Solved Examples: Electrochemistry | Physical Chemistry for NEET PDF Download
Solved Example
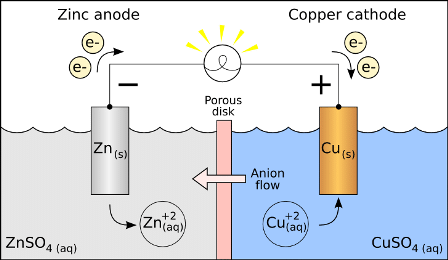 Galvanic Cell
Galvanic Cell
Ex.1 The reaction : Zn2+ (aq) + 2e- → Zn (s) has a electrode potential of - 0.76 V. This means-
(A) Zn cannot replace hydrogen from acids
(B) Zn is reducing agent
(C) Zn is oxidizing agent
(D) Zn2+ is a reducing agent
Sol. (B) Negative electrode potential shows that Zn2+ is difficult to be reduced and therefore, Zn acts as reducing agent.
Ex.2 Certain quantity of current is passed through 2V connected in series and containing XSO4(aq) and Y2SO4(aq) respectively. If the atomic masses of X and Y are in the ratio of 2 : 1 the ratio of the masses of Y liberated to that of X is :
(A) 1 : 1
(B) 1 : 2
(C) 2 : 1
(D) 3 : 2
Sol. (A) X2+ + 2e- → X ; Y+ + e-→ Y
2 mol e- produce X = 1 mol = 1 × M g
2 mol e- produce Y = 2 mol = 2 × M/2 = M g
Hence, ratio of the masses of Y:X is M:M or 1:1
Ex.3 The equivalent conductivities at infinite dilution of the cation and the anion of a salt A2B are 140 and 80 ohm-1 cm2 eq-1 respectively. The equivalent conductivity of the salt at infinite dilution is-
(A) 160 ohm-1 cm2 eq-1
(B) 220 ohm-1 cm2 eq-1
(C) 60 ohm-1 cm2 eq-1
(D) 360 ohm-1 cm2 eq-1
Sol. (B) (A2B) =
(A )
(B2-)
= 140 + 80 = 220 ohm-1 cm2 eq-1
Ex.4 The specific conductance of a 0.20 mol L-1 solution of an electrolyte at 20ºC is 2.48 x 10-4 ohm-1 cm-1. The molar conductivity of the solution is -
(A) 1.24 ohm-1 cm2 mol-1
(B) 4.96 ohm-1 cm2 mol-1
(C) 1.24 ohm-1 cm2
(D) 4.96 ohm-1 cm2
Sol. (A) Lm = =
= 1.24 ohm-1 cm2 mol-1
Ex.5 When an electric current is passed through acidulated, water, 112 mL of hydrogen gas at N.T.P. collects at the cathode in 965 seconds. The current passed, in amperes, is-
(A) 1.0
(B) 0.5
(C) 0.1
(D) 2.0
Sol. (A) 22,400 mL of hydrogen at STP(or NTP)=2g
Therefore,112 mL of hydrogen at
STP = = 10-2 g
Therefore,2H 2e- → H2
2F 1 mol
= 2 × 96,500 C = 2 g, 2 g hydrogen is deposited by 2 × 96,500 C
Therefore,10-2 g hydrogen will be deposited by = = 965 C
Q = i × t = 965 = i × 965
= i = 1
Ex.6 The charge required to deposit 40.5 g of Al (atomic mass = 27.0 g) from the fused Al2 (SO4)3 is-
(A) 4.34 × 105 C
(B) 43.4 × 105C
(C) 1.44 × 105
(D) None of these
Sol. (A) Al3+ + 3e- → Al
3F 1 mol = 27.0 g
to deposite 27g required charge =3×96,500 C
Therefore,to deposite 40.4g required charge
= = 4.34 × 105 C
Ex.7 The same amount of electricity was passed through two separate electrolytic cells containing solutions of nickel nitrate and chromium nitrate respectively. If 0.3 g of nickel was deposited in the first cell, the amount of chromium deposited is (At. wt. Ni= 59, Cr=52)
(A) 0.1 g
(B) 0.176 g
(C) 0.3 g
(D) 0.6 g
Sol. (B) =
For Ni2+ and Cr3+ , we have : =
mCr =
= 0.176 g
Ex.8 Electrolytic conduction differs from metallic conduction. In case of metallic conduction -
(A) The resistance increases with increasing temperature
(B) The resistance decreases with increasing temperature
(C) The flow of current does not generate heat
(D) The resistance is independent of the length of electrolytic conductor
Sol. (A) With increase in temperature vibration of Kernal (Cation) increases and therefore, conduction decreases and hence, resistance of the metallic conductor increases.
Ex.9 Three faraday of electricity is passed through molten solutions of AgNO3, NiSO4 and CrCl3 kept in three vessels using inert electrodes. The ratio in mol in which the metals Ag, Ni and Cr will be deposited is-
(A) 1 : 2 : 3
(B) 3 : 2 : 1
(C) 6 : 3 : 2
(D) 2 : 3 : 6
Sol. (C)
(i) Ag (aq) + e- → Ag (s)
1 mol = 1F 1 mol
3F = 3 mol
(ii)
Ni2+ (aq) + 2e- → Ni (s)
2 mol = 2F 1 mol
3 F =3/2 mol
(iii)
Cr3+ (aq) + 3e- → Cr(s)
3 mol = 3F 1 mol
The required ratio of moles of Ag, Ni and Cr is :
3 mol Ag : 3/2 mol Ni : 1 mol Cr
or 6 mol Ag : 3 mol Ni : 2 mol Cr.
Ex.10 In the reaction :
4 Fe + 3O2 → 4 Fe3+ + 6O2-
which of the following statements is correct
(A) A redox reaction
(B) O2 is reducing agent
(C) Fe3+ is an oxidizing agent
(D) Fe is reduced to Fe3+
Sol. (A)
In this reaction, Fe is oxidized to Fe3+ and O2 is reduced to O2_.
Ex.11 Calculate current strength in ampere required to deposit 10 g Zn in 2hrs. At wt. of Zn = 65.
Sol. Q w = ; Therefore,i =
i =
= 4.12 ampere
Ex.12 How many hour are required for a current of 3.0 ampere to decompose 18 g water.
Sol. H2O → H2 O2
Therefore,Eq. of H2O = Equivalent weight of H2O = 18/2 as two electrons are used for
1 mole H2O to decompose in H2 and O2.
Therefore, =
Therefore,t = 64333.3 sec = 1072.2 minute = 17.87 hr
Ex.13 Calculate the Avogadro's number using the charge on the electron 1.60 × 10-19 C and the fact that 96500 C deposits 107.9 g silver from its solution.
Sol. Q 96500 coulomb deposits 107.9 g Ag
Here eq. wt = Atomic weight
because Ag is monovalent. Thus 96500 coulomb charge means charge on N electrons
where N in Av. no.
Thus N × e = 96500
N =
= 6.03 × 1023 electrons
Ex.14 Calculate the volume of gases liberated at anode and cathode at NTP from the electrolysis of Na2SO4(aq.) solution by a current of 2 ampere passed
for 10 minute.
Sol. At cathode : 2H2O + 2e → H2 + 2OH-
At anode : 2H2O → 4H+ + 4e- + O2
Therefore,At anode =
= 8 Therefore,
=
=
= 0.0995 g
At NTP : Volume of O2 = = 0.0696 litre
Similarly at cathode =
=
= 0.0124 g
At NTP : Volume of H2 = = 0.139 litre
Ex.15 Fused Ni(NO3)2 is electrolysed between platinum electrodes using a current of 5 ampere for 20 minute. What mass of Ni is deposited at the cathode ?
Sol. Eq. of Ni deposited
=
=
= 0.0622
or wNi = 0.0622 × 58.71/2 = 1.825 g
(Ni2+ + 2e → Ni)
Ex.16 A current of 3.7 ampere is passed for 6 hrs. between Ni electrodes in 0.5
litre of 2M solution of Ni(NO3)2. What will be the molarity of solution at the
end of electrolysis ?
Sol. The electrolysis of Ni(NO3)2 in presence of Ni electrode will bring in following changes :
At anode : Ni → Ni2+ + 2e
At cathode : Ni2+ + 2e → Ni
Eq. of Ni2+ formed = Eq. of Ni2+ lost
Thus, there will be no change in conc. of Ni(NO3)2 solution during electrolysis i.e. ,
It will remain 2M
Ex.17 How long a current of 3 ampere has to be passed through a solution of AgNO3 to coat a metal surface of 80 cm2 with a thickness of 0.005 mm ?Density of Ag is 10.5 g cm-3
Sol. Given , i = 3 ampere
Also Volume covered by
Ag = 80 × 0.005 × 10-1 cm3 = 0.04 cm3
Therefore,Weight of Ag used = 0.04 × 10.5 g
wAg =
0.04 × 10.5 =
Therefore,t = 125.09 sec.
Ex.18 Calculate e.m.f of half cells given below :
(a) EºOP = 0 V
(b) EºOP = 0.44 V
(c) EºOP =- 1.36 V
Sol. (a) H2 → 2H+ + 2e
Therefore,EOP = EºOP- log
[H = 0.02 × 2M]
= 0- log
= 0.100 V
(b) Fe → Fe2 2e Therefore,EOP = EºOP- log [Fe2 ]
= 0.44- log[0.2]
= 0.4606 volt.
(c) 2Cl- → Cl2 2e Therefore,EOP = EºOP- log
=-1.36- log
=- 1.49 volt
Ex.19 If the oxidation of oxalic acid by acidified MnO4- solution were carried out in a reversible cell, what would be the electrode reaction ? Also calculate the equilibrium constant of the reaction. Given
= 1.51 V and
=- 0.49 V.
Sol. = 1.51 V
=- 1.51 V
=- 0.49 V
= 0.49 V
More is EºOP, more is the tendency to get oxidise
→ 2CO2 2e; EºOP = 0.49 v
8H 5e → Mn2 4H2O; EºRP = 1.51 V
16H → 10CO2 2Mn 8H2O; n = 10 Q 10 electron are used in redox change.
Therefore,EºCell = EºOP EºRP = 0.49 1.51 = 2.0 V Also Eº = log KC
Therefore,2 = log KC Therefore,KC = 10338.98
Ex.20 The e.m.f. of cell, Ag|AgI(s), 0.05 MKI || 0.05 M AgNO3 | Ag, is 0.788 V.
Sol. Ksp of AgI = [Ag ] [I-] = [Ag ] [0.05]
For given cell ECell = EOPAg ERPAg ….(i)
= -
log [Ag ]L.H.S.
log[Ag ]R.H.S. ECell =
log
0.788 = log
Therefore,[Ag ]L.H.S. = 2.203 × 10-15
By equation (i) Ksp = [2.203 × 10-15] [0.05] = 1.10 × 10-16
Ex.21 Calculate the reduction potential of a half cell consisting of a platinum electrode immersed in 2.0 MFe2 and 0.02 M Fe3 solution. Given = 0.771 V.
Sol. The half cell reaction is : Fe3+ + e → Fe2 (or take Fe2+ → Fe3+ + e)
Thus =
log
Therefore,= 0.771 +
log
= 0.771 +
log 10-2 = 0.653 V
Ex.22 An electrochemical cell has two half cell reactions as, A2 2e- → A; E° = 0.34 V, X → X2 + 2e- ; E° = 2.37 V. The cell voltage will be
(A) 2.71 V
(B) 2.03 V
(C) - 2.71 V
(D) - 2.03 V
Sol. (A) Ecell = 0.34 2.37 = 2.71 V
Ex.23 Eº of some oxidants are given as :
I2 + 2e → 2I- , Eº = 0.54 V
MnO4- 8H+ + 5e → Mn2+ + 4H2O, Eº= 1.52V
Fe3+ + e → Fe2+ ,Eº = 0.77V
Sn4+ + 2e → Sn2+ Eº = 0.1 V
(a) Select the strongest reductant and oxidant in these.
(b) Select the weakest reductant and oxidant in these.
(c) Select the spontaneous reaction from the changes given below :
(i) Sn4+ + 2Fe2+ → Sn2+ + 2Fe3+
(ii) 2Fe2+ + I2 → 2Fe3+ + 2I-
(iii) Sn4+ + 2I- → Sn2+ + I2
(iv) Sn2+ + I2 → Sn4+ + 2I-
Sol. (a) More or ve the EºOP, more is the tendency for oxidation or stronger is reductant.
Therefore, since maximum EºOP stands for
Sn2+ → Sn4+ + 2e , EºOP =-0.1 V
Therefore,strongest reductant : Sn2+ , and weakest oxidant : Sn4+
(b) More or ve is EºRP, more is the tendency for reduction or stronger is oxidant.
Therefore, since maximum EºRP stands for :
MnO4- + 8H+ + 5e → Mn2+ + 4H2O, EºRP = 1.52 V
Therefore,strongest oxidant : MnO4- , and weakest reductant: Mn2+
Note : Stronger is oxidant, weaker is its conjugate reductant and vice-versa.
(c) For (i) EºCell =
=-0.77 + 0.1
Fe2+ oxidizes and Sn4+ reduces in change, EºCell =-0.67 V, EºCell is negative
Therefore,(i) Is non-spontaneous change
For (ii) EºCell = =-0.77 0.54 =- 0.23 V
Therefore,(ii) Is non-spontaneous change
For (iii) EºCell = =-0.54 0.1 =-0.44 V
Therefore,(iii) Is non-spontaneous change
For (iv) EºCell = =-0.1 0.54 = 0.44 V
(iv) Is spontaneous change
Ex.24 Calculate the standard cell potentials of galvanic cell in which the following reactions take place :(Given EºOP Cr, Cd, Fe2+ , Ag are 0.74V, 0.40
V,- 0.77 V and- 0.80 V respectively)
(a) 2Cr(s) + 3Cd2+ (aq.) → 2Cr3+ (aq) + 3Cd
(b) Fe2+(aq) + Ag (aq.) → Fe3+ (aq.) + Ag(s)
Calculate the Dr,Gº and equilibrium constant of the reactions.
Sol. (a) EºCell =
[2Cr → 2Cr3+ + 6e; 3Cd2+ + 6e → 3Cd]
= 0.74 - 0.40) = 0.34 V
Six electrons (n = 6) are used in redox change
-ΔrGº=nEºF = 6 × 0.34 × 96500 J = 196860 J
or ΔrGº =-196.86 kJ
Also-ΔrGº = 2.303 RT log K
Therefore,196860 = 2.303 × 8.314 × 298 log K
K = 3.17 × 1034
(b) EºCell =
[Fe2 → Fe3 e; Ag e → Ag]
= -0.77 + 0.80 = 0.03 V
Also -ΔrGº = nEºF = 1 × 0.03 × 96500
or ΔrGº =-2895 J
Also -ΔrGº = 2.303 RT log K
2895 = 2.303 × 8.314 × 298 log K
K= 3.22
Ex.25 A cell is constituted as follows Pt, H2 (1 atm) | HA1 | HA2 | H2 (1 atm), Pt
The pH of two acids solutions HA1 and HA2 are 5 and 3 respectively. The emf of the cell is
(A) 0.059 V
(B) 0.0295 V
(C) 0.118 V
(D)- 0.118 V
Sol. (C)pH1 = 3 Therefore,[H ]c = 1 × 10-3 M ; pH2 = 5 Therefore,[H ]a = 1 × 10-5
Now, Ecell = log
= 0.059 × 2 = 0.118 V
Ex.26 The standard EMF of the cell reaction
Cu(s) +
Cl2(g) →
Cu2+ + Cl- is 1.02 V. The value of DG° will be
(A) unpredictable
(B)- 98.43 kJ
(C)- 196.86 kJ
(D)- 98.43 J
Sol. (B) ΔG° =- nFE° = - 1 × 96500 × 1.02
=- 98430 J
or =- 98.43 kJ
Ex.27 A current of 1.70 ampere is passed through 300 mL of 0.160 M solution of ZnSO4 for 230 sec with a current efficiency of 90%. Find the molarity of Zn2 after the deposition of Zn. Assume the volume of the solution remains constant during electrolysis.
Sol. We know, i = ampere
Therefore,Eq. of Zn2+ lost = =
= 3.646 × 10-3
Therefore,Meq. of Zn2+ lost = 3.646
Initial Meq. of Zn2+ = 300 × 0.160 × 2
[M × 2 = N for Zn2+ , Meq. = N × V(in mL)] = 48 × 2 = 96
Therefore,Meq. of Zn2+ left in solution = 96- 3.646 = 92.354
Therefore,[ZnSO4] = = 0.154 M
Ex.28 If 0.01 M solution of an electrolyte has a resistance of 40 ohms in a cell having a cell constant of 0.4 cm-1 then its molar conductance in ohm-1 cm2 mol-1 will be :
(A) 104
(B) 103
(C) 102
(D) 10
Sol. (B) K = × cell const. =
= 10-2
Lm = = 1000 = 103 ohm-1 cm2 mol-1
Ex.29 Calculate the quantity of electricity that would be required to reduce 12.3 g of nitrobenzene to aniline, if current efficiency is 50%. If the potential drops across the cell is 3.0 volt, how much energy will be consumed
Sol. C6H5NO2 + 6H+ + 6e → C6H5NH2 + 2H2O
N3+ + 6e → N3-
Therefore,Eq. wt. of nitrobenzene = =
, Since current efficiency is 50% Therefore,i =
Now w = 12.3 =
Therefore,i × t = 115800 coulomb
Now energy used = Q × V = 115800 × 3 = 347.4 kJ
Ex.30 A cell is containing two H electrodes. The negative electrode is in contact with a solution of 10-6 M H ion. The e.m.f. of the cell is 0.118 volt at 25ºC. Calculate [H ] at positive electrode.
Sol. Anode : H2 → 2H+ + 2e
(negative polarity) [H ] = 10-6 M
Cathode : 2H+ + 2e → H2
(positive polarity). [H ] → aM
Therefore,Ecell = =
-
log10[H ] 2Anode
log10[H ] 2Cathode
= log10
, 0.118=
log10
=
log10
, Therefore,[H ]Cathode = 10-4 M
Ex.31 A current of 2.0 A passed for 5 hours through a molten metal salt deposits 22.2 g of metal (At wt. = 177). The oxidation state of the metal in the metal salt is :
(A) 1
(B) 2
(C) 3
(D) 4
Sol. (C) E = × 96500 =
× 96500 = 59.5
Oxi. state = = 3
Ex.32 Cost of electricity for the production of x L H2 at NTP at cathode is Rs x; then cost of production of x L O2 at NTP at anode will be (assume 1 mole of electron as one unit of electricity)
(A) 2x
(B) 4x
(C) 16x
(D) 32x
Sol. (A) =
,
=
= 2, Volume of O2 =
Thus, L O2 requires Rs x for its production. i.e., x L O2 will require Rs x for its production.
Ex.33 In which direction can the reaction, 2Hg(l) + 2Ag+ (aq.) 2Ag(s) + Hg22+ (aq.) proceed spontaneously at the following concentrations of the ions participating in the reactions (i) and (ii) ?
(i) [Ag+ ] = 10-4 mol L-1 and [Hg22+ ] = 10-1 mol L-1
(ii) [Ag+]=10-1 mol L-1 and [Hg22+ ] = 10-4 mol L-1
Given : = 0.79 V;
= 0.80 V
Sol. (i) Q = =
= 107, Eº =
-
= 0.80- 0.79 = 0.01 V
E = Eº- log Q = 0.01-
log 107 =- 0.1965 V
Negative value shows that the reaction will proceed from right to left, i.e. in backward direction.
(ii) Q = =
= 10-2, Eº = 0.01 volt, E = Eº-
log10 Q = 0.01-
log10 10-2
= 0.01 0.059 V = 0.069 V
Since, the value of cell potential is positive, the reaction will proceed spontaneously in forward direction.
Ex.34 Two students use same stock solution of ZnSO4 and a solutions of CuSO4. The e.m.f. of one cell is 0.03 V higher than the other. The conc. of CuSO4 in the cell with higher e.m.f. value is 0.5 M. Find out the conc. of CuSO4 in the other cell [(2.303 RT/F) = 0.06].
Sol. As given,
Cell I : ZnCu, Ecell =
log
….(i)
Cell II : ZnCu E¢cell =
log
….(ii)
If Ecell>E¢cell , then Ecell-E¢cell=0.03 V and C2=0.5 M
By Eq. (i) and (ii)
Ecell- E¢cell = log
, 0.03 =
log
, C¢2 = 0.05 M
Ex.35 How much will the reduction potential of a hydrogen electrode change when its solution initially pH = 0 is neutralized to pH = 7.
(A) Increase by 0.059 V
(B) Decrease by 0.058 V
(C) Increase by 0.41 V
(D) Decrease by 0.41 V
Sol. (D) Eº = E log 10-7 = Eº
= Eº- 0.41 V
Ex.36 The time required to coat a metal surface of 80 cm2 with 5 × 10-3 cm thick layer of silver (density 1.05 g cm-3) with the passage of 3A current through a silver nitrate solution is.
(A) 115 sec
(B) 125 sec
(C) 135 sec
(D) 145 sec
Sol. (B) Weight of Ag required
= 80 × 5 × 10-3 × 1.05 (wt. = V × d) = 0.42 g
Q w = Therefore,0.42 =
Therefore,t = 125 sec
Ex.37 Standard electrode potentials are
Fe2+/Fe (E° =- 0.44 V), Fe3+ /Fe2+ (E° = 0.77 V)
Fe2+, Fe3+ and Fe blocks are kept together, then :
(A) Fe3+ increases
(B) Fe3+ decreases
(C) Fe2+ /Fe3+ remains unchanged
(D) Fe2+ decreases
Sol. (B) Fe 2+ → Fe + Fe+3, =- 0.44- 0.77 = -0.121
So spontaneous reaction is Fe + Fe3+ → Fe 2+
Ex.38 Conductivity (Unit : siemen's S) is directly proportionally to the area of the vessel and the concentration of the solution in it and is inversely proportional to the length of vessel, then the unit of constant of proportionality is
(A) S m mol-1
(B) S m2 mol-1
(C) S-2 m2 mol
(D) S2 m2 mol-2
Sol. (B)
Ex.39 Passage of three faraday of charge through aqueous solution of AgNO3, CuSO4, Al(NO3)3 and NaCl respectively will deposit the metals in the ratio (molar).
(A) 1 : 2 : 3 : 1
(B) 6 : 3 : 2 : 6
(C) 6 : 3 : 0 : 0
(D) 3 : 2 : 1 : 0
Sol. (C) 3 eq. of Ag and Cu, zero equivalent of Na and Al will be deposited.
Note electrolysis of NaClaq. and AlCl3aq. does not give Na and Al metal at cathode.
Thus molar ratio is : for Ag : Cu
or 3 : or 6 : 3 : 0 : 0 for Ag : Cu : Al : Na
Ex.40 and
are -0.441 V and 0.771 V respectively, Eº for the reaction.
Fe + 2Fe3+ → 3Fe2+ , will be.
(A) 1.212 V
(B) 0.111 V
(C) 0.330 V
(D) 1.653 V
Sol. (A) =
= 0.441 + 0.771 = 1.212 V
Ex.41 Efficiency of a cell with cell reaction under standard conditions,
A(s) + B+ → A+ + B(s); DHº =-300 kJ is 70%. The standard electrode potential of cell is.
(A) 2.176 V
(B) 2.876 V
(C) 1.248 V
(D) 1.648 V
Sol. (A) Efficiency = =
, Eº =
=
= 2.176 V
Ex.42 Eº for F2 2e 2F- is 2.7 V. Thus Eº for F-
F2 e is.
(A) 1.35 V
(B)- 1.35 V
(C)- 2.7 V
(D) 2.7 V
Sol. (C) EºOP =- EºRP
Ex.43 Salts of A (atomic weight 7). B (atomic weight 27) and C (atomic weight 48) were electrolysed under identical condition using the same quantity of electricity. It was found that when 2.1 g of A was deposited, the weights of B and C deposited were 2.7 and 7.2 g. The valencies of A, B and C respectively.
(A) 3, 1 and 2
(B) 2, 6 and 3
(C) 3, 1 and 3
(D) 2, 3 and 2
Sol. (B) gm EqA = gm Eq B = gm EqC
, 0.3 x = 0.1 y = 0.15 z
3x = y = 1.5z
x = 2, y = 6, z = 3
Ex.44 Standard electrode potential data are useful for understanding the suitability of an oxidant in a redox titration. Some half cell reactions and their standard potentials are given below: + 8H +(aq.) + 5e →
Mn2+ (aq.) + 4H2O(l); Eº = 1.51 V + 14H +(aq.) + 6e →
2Cr3+ (aq.) + 7H2O(l) ; Eº = 1.38 V
Fe3+(aq.) + e- → Fe2+(aq.); Eº = 0.71 V
Cl2(g) + 2e- → 2C-(aq.); Eº = 1.40 V
Identify the only incorrect statement regarding the quantitative estimation of
aqueous Fe(NO3)2.
(A) MnO4- can be used in aqueous HCl
(B) Cr2O72_ can be used in aqueous HCl
(C) MnO4- can be used in aqueous H2SO4
(D) Cr2O72_ can be used in aqueous H2SO4
Sol. (A) MnO4- will oxidise Cl- ion according to equation
Mn7+ + 5e → Mn2+ , 2Cl- → Cl2 + 2e,
Thus Eºcell = =-1.40 1.51 = 0.11 V
or reaction is feasible.
MnO4- will oxidize Fe2+ to Fe3+
 Mn7+ + 5e → Mn2+ , Fe3+ → Fe2+ + e, Eºcell =
Mn7+ + 5e → Mn2+ , Fe3+ → Fe2+ + e, Eºcell = = -0.77 + 1.51 = 0.74 V
or reaction is feasible
Thus MnO4- will not oxidize only Fe2+ to Fe3+ in aqueous HCl but it will also oxidise Cl- to Cl2. Suitable oxidant should not oxidise Cl- to Cl2 and should oxidise only Fe2+ to Fe3+ in redox titration.
Ex.45 The emf of the cell Zn | Zn2+ (0.01M)|| Fe2+ (0.001M)| Fe at 298 K is 0.2905, then the value of equilibrium constant for the cell reaction is.
(A) e0.32/0.0295
(B) 100.32/0.0295
(C) 100.26/0.0295
(D) 100.32/0.0591
Sol. (B) Zn + Fe2+ → Fe + Zn2+
Ecell = Eºcell + log
, 0.2905 = Eºcell +
log
Therefore,Eºcell = 0.2905 + 0.0295 = 0.32 V, Now Eºcell =log10 Kc , 0.32 =
log10 Kc
Therefore,Kc = 100.32/0.0295
Ex.46 The rusting of iron takes place as follows :
2H+ + 2e + ½O2 → H2O(l); Eº = 1.23 V
Fe2+ + 2e → Fe(s); Eº =-0.44 V
The ΔGº for the net process is.
(A)-322 kJ mol-1
(B)-161 kJ mol-1
(C)- 152 kJ mol-1
(D)-76 kJ mol-1
Sol. (A) Eºcell = +
= 0.44 +1.23 = 1.67 V
Therefore,ΔGº =-nEº F =-2 × 1.67 × 96500 J =-322.31 kJ mol-1
Passage : (Q.47 to 52)
Electrolysis involves electronation and de-electronation at the respective electrodes. Anode of electrolytic cell is the electrode at which de-electronation takes place whereas at cathode electronation is noticed. If two or more ions of same charge are to be electronated or de-electronated, the ion having lesser discharge potential is discharged. Discharge potential of an ion refers for EºOP or EºRP as the case may be. The products formed at either electrode is given in terms of Faraday's laws of electrolysis i.e., w =.
Ex.47 During electrolysis of CH3COONa(aq.), the mole ratio of gases formed at cathode and anode is.
(A) 1 : 2
(B) 2 : 1
(C) 3 : 1
(D) 1 : 3
Sol. (D) Anode :
2CH3COO- → C2H6 + 2CO2 + 2e (3 mole)
Cathode : 2H+ + 2e → H2 (1 mole)
Ex.48 During electrolysis of HCOONa(aq.), the gas liberated at anode and cathode are respectively.
(A) H2, CO2 and H2
(B) H2, CO2 and O2
(C) H2 and O2
(D) O2 and H2
Sol. (A) Anode : 2HCOO- → H2 + 2CO2 + 2e;
Cathode : 2H + + 2e → H2
Ex.49 During electrolysis of CuSO4(aq.), the pH of solution becomes.
(A) < 7
(B) > 7
(C) = 7
(D) ³ 7
Sol. (A) Anode : 2OH- → H2O + ½O2 + 2e;
Cathode : Cu2+ + 2e → Cu
[OH-] decreases, thus pH decrease i.e. < 7.
Ex.50 5 litre solution of 0.4 M CuSO4(aq.) is electrolysed using Cu electrode. A current of 482.5 ampere is passed for 4 minute. The concentration of CuSO4 left in solution is.
(A) 0.16 M
(B) 0.32 M
(C) 0.34 M
(D) 0.40 M
Sol. (D) It is the case of attacked electrodes that is
Anode : Cu → Cu2++ 2e;
Cathode : Cu2+ + 2e → Cu
Thus no change in conc. of Cu2 ions.
Ex.51 5 litre solution of 0.4 M Ni(NO3)2 is electrolysed using Pt electrodes with 2.4125 ampere current for 10 hour.
(A) 0.31 M
(B) 0.22 M
(C) 0.26 M
(D) 0.40 M
Sol. (A) Eq. of Ni(NO3)2 = 5 × 0.4 × 2 = 4;
Eq. of Ni2 lost = =
= 0.9
Therefore,Eq. of Ni(NO3)2 left = 4- 0.9 = 3.1, Therefore,Molarity of Ni(NO3)2 = = 0.31
Ex.52 The volume of octane required to be used for its combustion by the oxygen liberated during electrolysis of an NaNO3(aq.) by passing 9.65 ampere current for 1 hr. is.
(A) 322.56 mL
(B) 32.256 mL
(C) 3.22 mL
(D) 1.612 × 102 mL
Sol. (D) Anode : 2OH- → H2O+ ½O2 + 2e;
Cathode : 2H+ + 2e → H2
Eq. of O2 formed = = 0.36
Therefore,Mole of O2 formed = = 0.09, C8H18
O2 → 8CO2 9H2O
Therefore,Mole of C8H18 = × 0.09 = 7.2 × 10-3 , Therefore,
= 7.2 × 10-3 × 22400 161.2 mL
Ex.53 The specific conductivity of 0.02 M KCl solution at 25°C is 2.768 × 10-3 ohm-1 cm-1. The resistance of this solution at 25°C when measured with a particular cell was 250.2 ohm. The resistance of 0.01 M CuSO4 solution at 25°C measured with the same cell was 8331 ohm. Calculated the molar conductivity of the copper sulphate solution.
Sol. Cell constant = =
= 2.768 × 10-3 × 250.2
For 0.01 M CuSO4 solution
Sp. conductivity = Cell constant × Conductance = 2.768 × 10-3 × 250.2 ×
Molar conductance = Sp. cond. × =
×
=8.312 ohm-1 cm2 mol-1
Ex.54 A decinormal solution of NaCl has specific conductivity equal to 0.0092. If ionic conductances of Na and Cl- ions at the same temperature are 43.0 and 65.0 ohm-1 respectively. Calculate the degree of dissociation of NaCl solution
Sol. Equivalent conductance of N/10 NaCl solution = Sp. conductivity × dilution = 0.0092 × 10,000 = 92 ohm-1
=
= 43.0 65.0 = 108 ohm-1
Degree of dissociation, a = = 0.85
Ex.55 The specific conductance of saturated solution of AgCl is found to be 1.86 × 10-6 ohm-1 cm-1 and that of water is 6 × 10-8 ohm-1 cm-1. The solubility of AgCl is ….
Given, = 137.2 ohm-1 cm2 eq-1
(A) 1.7 × 10-3 M (B) 1.3 × 10-5 M (C) 1.3 × 10-4 M (D) 1.3 × 10-6 M
Sol. kAgCl = kAgCl (Solution) = 1.86×10-6-6×10-8=1.8 × 10-6 ohm-1 cm-1
, S=
=1.31×10-5 M
Ex.56 Resistance of a solution (A) is 50 ohm and that of solution (B) is 100 ohm, both solution being taken in the same conductivity cell. If equal volumes of solution (A) and (B) are mixed, what will be the resistance of the mixture, using the same cell ? Assume that there is no increase in the degree of dissociation of (A) and (B) mixing
Sol. Let us suppose k1 and k2 are the specific conductance of solutions `A' and `B' respectively and cell constant is `y'. We know that,
Specific conductance=Conductance × Cell constant
For (A), For (B),
When equal volume of (A) and (B) are mixed, the volume becomes double. Then, Specific conductance of mixture =
Therefore,
R = 200/3 = 66.66 ohm
Ex.57 A big irregular shaped vessel contained water, specific conductance of which was 2.56 × 10-5 mho cm-1, 500 g of NaCl was then added to the water and the specific conductance after the addition of NaCl was found to be 3.1 × 10-5 mho cm-1. Find the capacity of the vessel if it was fully filled with water. ( NaCl = 149.9 ohm-1 cm2 eq-1)
Sol. Let us suppose the volume of vessel is V mL
Volume containing 1 equivalent = =
Specific conductance of NaCl = Specific conductance of NaCl solution- Specific conductance of water
= 3.1 × 10-5- 2.56 × 10-5 = 0.54 × 10-5 mho cm-1 = k × volume containing 1 equivalent of electrolyte ….(i)
For very dilute solution, when the big vessel is fully filled = 149.9 ohm-1 cm2 eq-1 , Thus, from eq. (i) 149.9 = 0.54 × 10-5 ×
V = 237258.38 L
Ex.58 Maximum Conductivity would be of-
(A) K3Fe(CN)6 [0.1 M solution]
(B) K2Ni(CN)4 [0.1M solution]
(C) FeSO4.Al2(SO4) 3.24H2O [0.1 M solution]
(D) Na[Ag(S2O2)3] [0.1 M solution]
Sol. (C)
Double salt on ionization gives more ions. One molecule of the salt gives Fe 2, 2Al 3, 4SO4-2 ions. Hence its conductance would be highest.
Discussion Questions
(i) For the reaction given below, apply Le-Chatelier principle to justify the results recorded by you and also bring out mathematical rationalisation of your results.
Zn(s) Cu2 (aq) Zn2 (aq) Cu(s),
(ii) Determine the slope of the graph. Match experimental value with the theoretical value. On what factors does the value of slope depend?
(iii) Devise another experiment to study the variation in cell potential with concentration of one of the ions involved in a cell reaction.
(iv) What factor is kept in mind while selecting an electrolytic solution for the construction of a salt bridge?
(v) Is it possible to measure the single electrode potential?
|
117 videos|223 docs|237 tests
|
FAQs on Solved Examples: Electrochemistry - Physical Chemistry for NEET
| 1. What is electrochemistry? |  |
| 2. What is an electrochemical cell? |  |
| 3. What is the difference between oxidation and reduction? |  |
| 4. What is the significance of standard electrode potential? |  |
| 5. What are some applications of electrochemistry? |  |






















