Chapter : Atomic Spectroscopy, PPT, Semester, Engineering - Electronics and Communication Engineering (ECE) PDF Download
Atomic Spectroscopy:
Atomic Emission Spectroscopy
Atomic Absorption Spectroscopy
Atomic Fluorescence Spectroscopy
* Elemental Analysis
* Sample is atomized
* Absorption, emission or fluorescence of atoms or ions in the gas phase
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Atomic Emission Spectroscopy
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
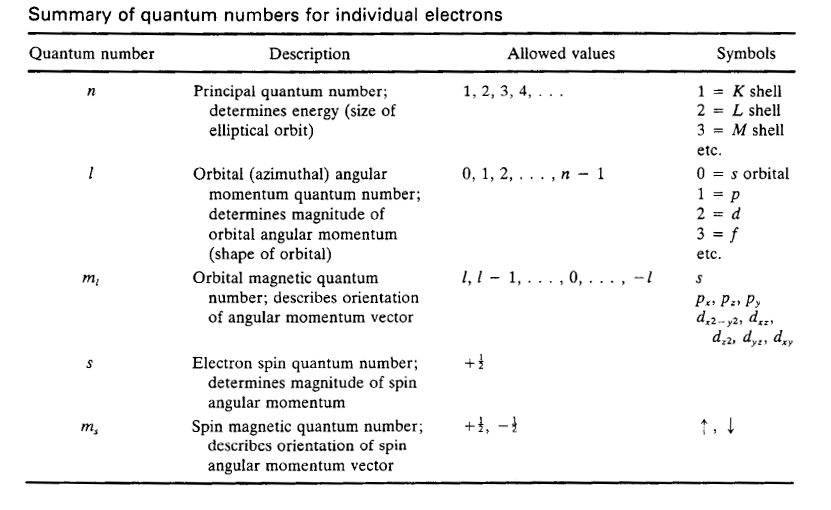
Electronic Levels for Individual Electrons
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
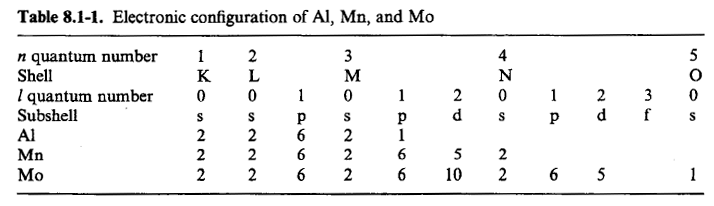
Electronic Configuration of Atoms
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
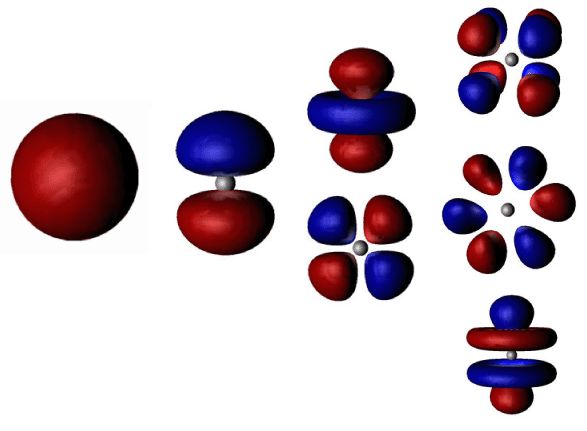
Electronic Configuration of Atoms
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
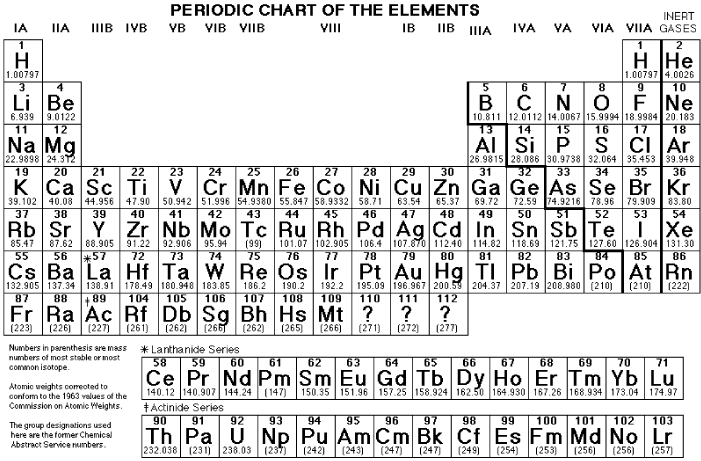
Electronic Configuration of Atoms
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Electronic States of Atoms
Quantum numbers for electronsQuantum numbers for many-electron atoms
l: orbital angular momentum quantumL: orbital angular momentum quantum number
number (0,1, … n-1 e.g., for 2 e-: L= l1+l2, l1+l2-1, l1+l2-2, …,| l1-l2|
where 0=s, 1=p, 2=d, 3=f)0 = S, 1 = P, 2 = D, 3 = F
ml: orbital magnetic quantum numberML: orbital magnetic quantum number (Σml)
(l, l-1, …, 0, …, -l )2L+1 possible values
s: electron spin quantum number (1/2) S: total spin quantum number
S = s1+s2, s1+s2 -1, …,| s1-s2 |
S = 0 singlet, S = 1 doublet, S = 2 triplet
ms: spin magnetic quantum numberMS: spin magnetic quantum number (Σms)
(+1/2, -1/2)2S+1 possible values
J: total angular quantum number
J = L+S, L+S-1, …, | L-S|
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Spectroscopic Description of Atomic Electronic States –Term Symbols
Multiplicity (2S +1)describes the number of possible orientations of total spin angular momentum whereS is the resultant spin
quantum number(1/2 x # unpaired electrons)Resultant Angular Momentum (L)describes the coupling of the orbital angular momenta of each electron (add the mLvalues for each electron)Total Angular Momentum (J)combines orbital angular momentum and intrinsic
angular momentum (i.e., spin).To Assign J Value:if less than half of the subshell is occupied, take the minimum value J = | L − S | ; if more than half-filled, take the maximum value J = L + S; if the subshell is half-filled, L = 0 and then J = S.
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Spectroscopic Description of Ground Electronic States –Term Symbols
Term Symbol Form: 2S+1{L}J2S+1 –multiplicityL –resultant angular momentum quantum numberJ –total angular momentum quantum numberGround state has maximal S and L values. Example: Ground State of Sodium –1s22s22p63s1Consider only the one valence electron (3s1)L = l = 0, S = s = ½, J = L + S = ½so, the term symbol is 2S½
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Are you getting the concept?
Write the ground state term symbol for fluorine.
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Spectroscopic Description of All Possible Electronic States –Term Symbols
C –1s22s22p2
Step 1:Consider two valence p electrons
1st2p electron has n = 2, l = 1, ml= 0, 1, ms= ½ → 6 possible sets of quantum numbers
2nd2p electron has 5 possible sets of quantum numbers (Pauli Exclusion Principle)
For both electrons, (6x5)/2 = 15 possible assignments since the electrons are indistinguishable
Step 2: Draw all possible microstates. Calculate ML and MSfor each state.
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Spectroscopic Description of All Possible Electronic States –Term Symbols
C –1s22s22p2
Step 3: Count the number of microstates for each ML—MSpossible combination
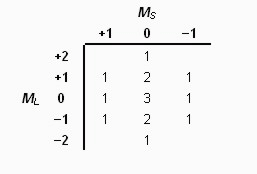
Step 4: Extract smaller tables representing each possible term
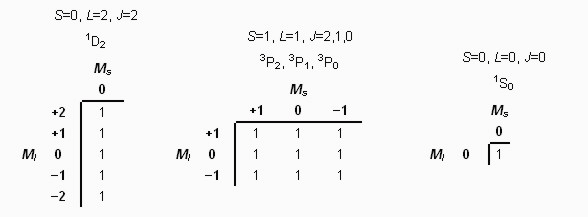
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Spectroscopic Description of All Possible Electronic States –Term Symbols
C –1s22s22p2
Step 5: Use Hund’s Rules to determine the relative energies of all possible states.1. The highest multiplicity term within a configuration is of lowest energy.2. For terms of the same multiplicity, the highest L value has the lowest energy (D < P < S).3. For subshells that are less than half-filled, the minimum J-value state is of lower energy than higher J-value states.4. For subshells that are more than half-filled, the state of maximum J-value is the lowest energy.Based on these rules, the ground electronic configuration for carbon has the following energy order: 3P0< 3P1< 3P2< 1D2< 1S0
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Spectroscopic Description of Excited States –Term Symbols
Write term symbols in analogous manner except consider the orbital to which an electron is promoted.
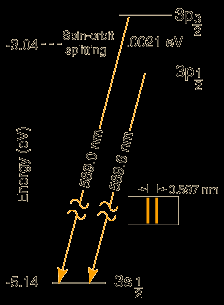
For example, excitation of Na promotes one valence electron into the 3p orbital. In this case, n = 3, S = ½, 2S+1 = 2, L = 1 (P term), J = 3/2, 1/2.
There are two closely spaced levels in the excited term of sodium with term symbols 2P1/2and 2P3/2
This type of splitting (same L but different J) is called fine structure.
Transition from 2P1/2→ 2S1/2
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Calculating Energies for Transitions
To calculate the energy of a single electron atomwith quantum numbers L, S, and J:EL,S,J= ½ hcλ[J(J+1) -L(L+1) –S(S+1)]where λ is the spin-orbit coupling constant
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Are you getting the concept?
Atomic emission spectra show a doublet in the Na spectrum due to spin-orbit coupling of the 2P state. Given that λ= 11.4 cm-1, find the energy spacing (in cm-1) between the upper 2P3/2and 2P1/2states.
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Allowed and Forbidden Transitions
Only a fraction of all possible transitions are observed.Allowed transitions-high probability, high intensity, electric dipole interactionForbidden transitions-low probability, weak intensity, non-electric dipole interactionSelection rules for allowed transitions:* The parity of the upper and lower level must be different. (The parity is even if Σliis even. The parity is odd if Σliis odd.)* Δl= ±1* Δj= 0 or ±1, but J= 0 to J= 0 is forbidden.
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Additional Splitting Effects
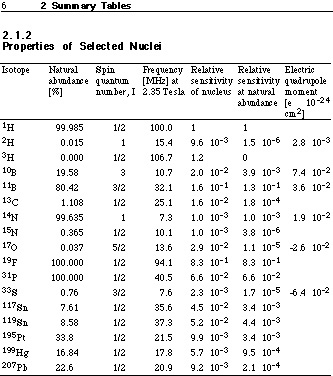
•Hyperfine splittingdue to magnetic coupling of spin and orbital motion of electrons with the nuclear spin.
•Isotope shift. Sufficient to determine isotope ratios.
•Splitting in an electric field (Stark effect): Relevant for arc and spark techniques.
•Splitting in a magnetic field (Zeeman effect):* In absence of a magnetic field, states that differ only by their MJvalues are degenerate, i.e., they have equivalent energies.* In presence of a magnetic field, this is not true anymore.* Can be used for background correction.
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Pretsch/Buhlmann/Affolter/Badertscher,Structure Determination of Organic Compounds
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Stark Splitting
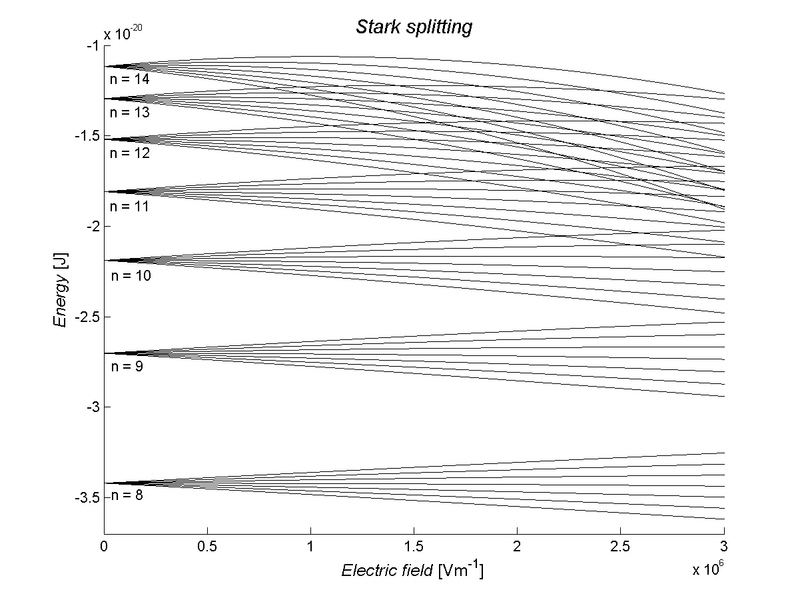
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Zeeman Splitting
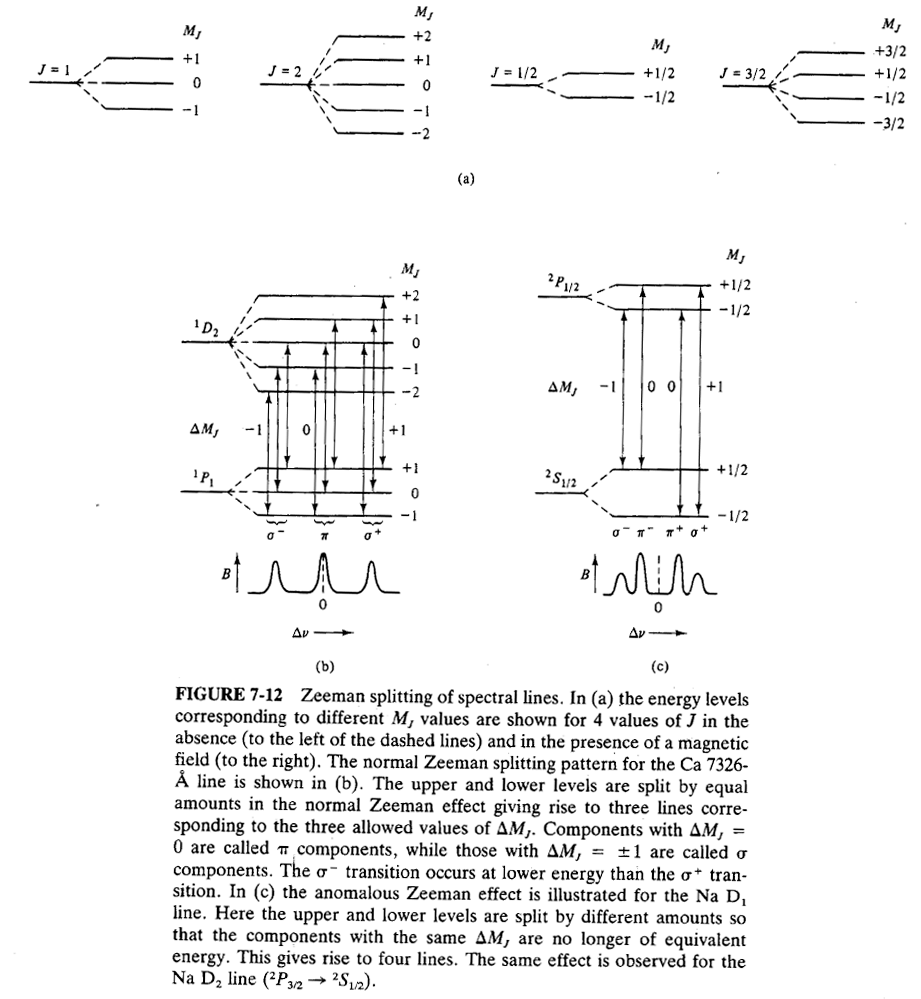
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Sample Introduction and Atomization
Atomization:Convert solution → vapor-phase free atomsMeasurements usually made in hot gas or enclosed furnace:
• flames
•plasmas
•electrical discharges (arcs, sparks)
•heated furnaces
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Atomic Emission Spectroscopy (AES)
See also: Fundamental reviews in Analytical Chemistry
e.g. Bings, N. H.; Bogaerts, A.; Broekaert, J. A. C. Anal. Chem.2002, 74, 2691-2712 (“Atomic Spectroscopy”)
•Beginning 19th century: alcohol flame (Brewster, Herschel, Talbot, Foucault)
•mid 1800s: Discovery of Cs, Tl, In, Ga by atomic spectroscopy (Bunsen, Kirchhoff)
•1877: Gouy designs pneumatic nebulizer
•1920s: Arcs and sparks used for AES
•1930s: First commercial AES spectrometer (Siemens-Zeiss)
•1960s: Plasma sources (commercial in 1970s)
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Atomic Emission Spectroscopy (AES)
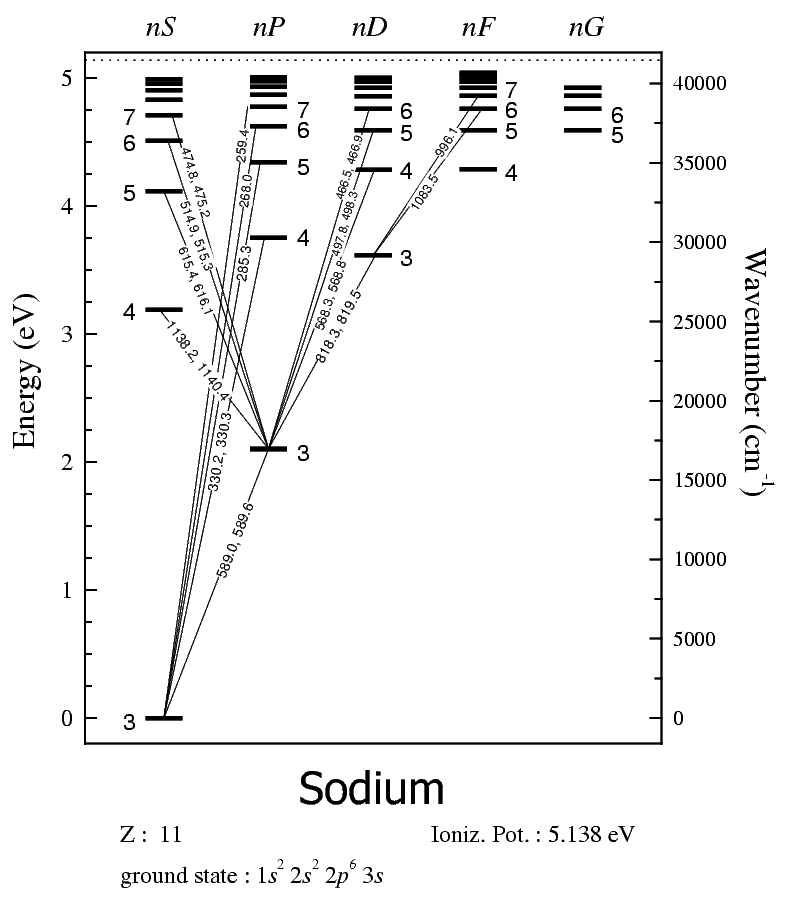 At RT, nearly allelectrons in 3sorbitalExcite with flame, electric arc, or sparkCommon electronictransitions
At RT, nearly allelectrons in 3sorbitalExcite with flame, electric arc, or sparkCommon electronictransitions
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Example AE Spectra
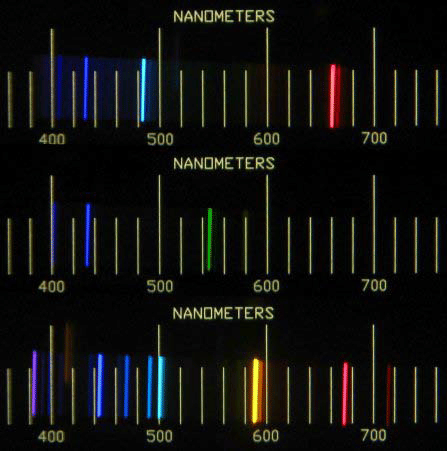
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
An Ideal AES Source
1.complete atomization of all elements
2.controllable excitation energy
3.sufficient excitation energy to excite all elements
4.inert chemical environment
5.no background
6.accepts solutions, gases, or solids
7.tolerant to various solution conditions and solvents
8.simultaneous multi-element analysis
9.reproducible atomization and excitation conditions
10.accurate and precise analytical results
11.inexpensive to maintain
12.ease of operation
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Flame AES
•Background signals due to flame fuel and oxidants –line spectra:
•OH•281.1, 306.4, 342.8 nmfrom O + H2⇒H + OHH + O2⇒O + OH
•O2250, 400 nm
•CH431.5, 390.0, 314.3 nm
•CObands between 205 to 245 nm
•CN, C2, CH, NH bands between 300 to 700 nmUnlike bands of atomic origin, these molecular bands are fairly broad.
•Continuum emission from recombination reactionse.g. H + OH ⇒H2O + hνCO + O ⇒CO2+ hν⇒⇒ Flames used in AES nowadays only for few elements. Cheap but limited. {Flame AES often replaced by flame AAS.}
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Inductively Coupled Plasma AES
•Spectral interference more likely for plasma than for flame due to larger population of energetically higher states.
•Modern ICP power: 1–5 kW (4 to 50 MHz)
•4000 to 10,000 K: Very few molecules
•Long residence time (2–3 ms)results in high desolvationand volatilization rate
•High electron density suppresses ionization interference effects
•Background: Ar atomic lines and,in hottest plasma region, Bremsstrahlung (continuum radiationfrom slowing of charged particles)
•Price > $ 50 k
•Operating cost relatively high dueto Ar cost (10–15 mL/min) andtraining.

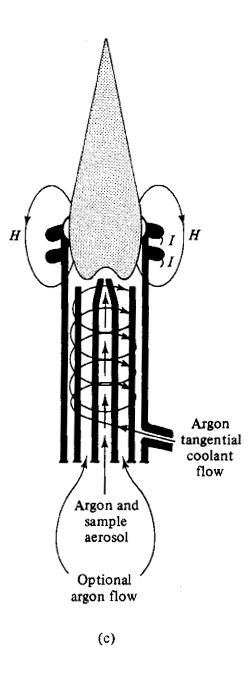
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
Microwave Plasma AES
•Power 25 to 1000 W (ICP 1000–2000 W)
•Frequency 2450 MHz (ICP 4 to 50 MHz)
•Argon, helium or nitrogen
•Temperature estimated to be 2000 -3000 K
•Low temperature causes problems with liquids
•Useful for gases: GC–microwave plasma AES
Atomic Spectroscopy----------------------------------------------------- Next Slide----------------------------------Ingle Crouch
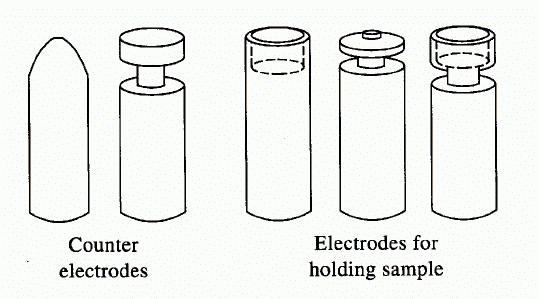
Arcs and Sparks
•Arc = An electrical discharge between two or more conducting electrodes (1-30 A)
•Spark = An intermittent high-voltage discharge (few μsec)
•Limited to qualitative and semi-quantitative use (arc flicker)
•Particularly useful for solid samples (pressed into electrode)
•The burn takes > 20 sec (need multichannel detector)
FAQs on Chapter : Atomic Spectroscopy, PPT, Semester, Engineering - Electronics and Communication Engineering (ECE)
| 1. What is atomic spectroscopy? |  |
| 2. How does atomic spectroscopy work? |  |
| 3. What are the different types of atomic spectroscopy techniques? |  |
| 4. What are the applications of atomic spectroscopy? |  |
| 5. What are the advantages of atomic spectroscopy? |  |



















