Chapter - Basics Of Corrosion, PPT, Semester, Engineering - Electronics and Communication Engineering (ECE) PDF Download
BASICS OF CORROSION
Dr. Ramazan Kahraman
Chemical Engineering Department
King Fahd University of Petroleum & Minerals
Dhahran, Saudi Arabia
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
What is Corrosion?
Reaction of a metal with its environment
♦ Aqueous corrosion
− reaction with water (usually containing
dissolved ions)
♦ High temperature oxidation
− reaction with oxygen at high temperature
♦ High temperature corrosion
− reaction with other gases
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Examples of Corrosion
Rusting of steel
–corrosion product (rust) is solid but not protective
Reaction of aluminium with water
–corrosion product is insoluble in water, so may be protective
Burning of magnesium in air
–high temperature oxidation

Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Corrosion Science and Engineering
♦Corrosion Science
– Study of the chemical and metallurgical processes that occur during corrosion.
♦Corrosion Engineering
– Design and application of methods to prevent corrosion.
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Why is Corrosion Happening?
Because metals want to go back to their stable states.
For Example, Fe is stable when it reacts with oxygen.
So, in the presence of a corrosive environment, Fe tends to separate (decompose) from steel and reacts with oxygen
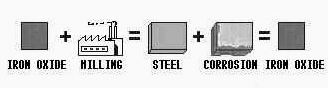
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Nature of Corrosion
Formation of cell is essential for corrosion Corrosion cell comprises of the following
–Anode (supplies e- - oxidation reaction)
–Cathode (consumes e- - reduction reaction)
–Electrolyte
–Conductor (electron path)
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Electrodes
Electrodes are pieces of metal on which an electrochemical reaction is occurring
An anode is an electrode on which an anodic or oxidation reaction is occurring
A cathode is an electrode on which a cathodic or reduction reaction is occurring
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Electrochemical Cell
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Electrochemical Cell (cont.)

Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Corrosion of a Metal in Acid
Anodic Rxn M M+n + n e-
Cathodic Rxn nH+ + n e- n/2 H2
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Corrosion of a Metal in Aerated Water or Aerated Basic Solutions
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Acids and Bases
An acid is a substance that produces excess hydrogen ions (H+) when dissolved in water
–examples are HCl, H2SO4
A base (alkali) is a substance that produces excess hydroxyl ions (OH-) when dissolved in water
–examples are NaOH, KOH
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Acids and Bases (cont.)
Note that H+ and OH- are in equilibrium in water:
H2O ⇔ H+ + OHThe
product of [H+] times [OH-] is 10-14, so in pure water both [H+] and [OH-] are 10-7. This leads to the concept of pH, which is defined as -log[H+]
Hence pH = 0 is strong acid, 7 is neutral, and 14 is strong alkali
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Corrosion of Zinc in Acid
● Zinc known as a base or active metal
● Zinc dissolves with hydrogen evolution
Zn + 2HCl → ZnCl2 + H2
But we can separate metal dissolution and hydrogen evolution
Zn → Zn2+ + 2e-
2H+ + 2e- → H2
These are known as electrochemical reactions
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Corrosion of Platinum in Acid
Platinum does not react with acids
Platinum is known as a noble metal
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Zinc and Platinum in Acid – Not Connected
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Connection of Platinum to Zinc
(This is galvanic corrosion which will be studied in detail later)
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
External Current Applied to Platinum in Acid
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
External Current Applied to Platinum in Alkali
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
External Current Applied to Platinum
Hydrogen evolution at one electrode
2H+ + 2e- → H2 (acids)
or 2H2O + 2e- → H2 + 2OH- (alkalis)
A piece of metal in the solution
Oxygen evolution at the other electrode
2H2O → O2 + 4H+ + 4e- (acids)
or 4OH- → O2 + 2H2O + 4e- (alkalis)
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Anodic Reactions
Oxidation reactions
Produce electrons
Examples
Zn → Zn2+ + 2e- zinc corrosion
Fe→ Fe2+ + 2e- iron corrosion
Al→ Al3+ + 3e- aluminium corrosion
Fe2+ → Fe3+ + e- ferrous ion oxidation
H2 → 2H+ + 2e- hydrogen oxidation in acids
H2 + 2OH- → 2H2O + 2e- hydrogen oxidation in water or bases
2H2O → O2 + 4H+ + 4e- oxygen evolution in acids
4OH- → O2 + 2H2O + 4e- oxygen evolution in water or bases
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Cathodic Reactions
Reduction reactions
Consume electrons
Examples
O2 + 2H2O + 4e-→ 4OH- oxygen reduction in water/bases
O2 + 4H+ + 4e- → 2H2O oxygen reduction in acids
2H2O + 2e-→ H2 + 2OH- hydrogen evolution in water/bases
2H+ + 2e- → H2 hydrogen evolution in acids
Cu2+ + 2e- → Cu copper plating
Fe3+ + e- → Fe2+ ferric ion reduction
Sn4+ + 2e- → Sn2+
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Cathodic Rxns in Acidic & Basic Solns
Deaerated Acidic Solutions
2H+ + 2e- → H2
Aerated Acidic Solutions
2H+ + 2e- → H2
O2 + 4H+ + 4e- → 2H2O
(presence of O2 further increases corrosion)
Deaerated Neutral or Basic Solutions
2H2O + 2e- → H2 + 2OH-
Aerated Neutral or Basic Solutions
O2 + 2H2O + 4e- → 4OH-
(this reaction causes higher corr. rate)
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Corrosion Rate
Simplest and most useful technique for corrosion rate determination is the Weight Loss Technique Corrosion Rate = mass / exposed surface area . time
or
Corrosion Rate = avg. corrosion penetration depth / time
( = mass / density . surface area . time )
Common Corrosion Rate Units
– gmd (grams of metal lost per square meter per day)
– mm/y (average millimeters penetration per year)
– mpy (avg. mils penetration per year, 1 mil = 0.001 in)
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Example
A carbon steel test specimen of dimensions 2-in × 3-in × 0.125-in with a 0.25-in hole for suspending in solution is exposed for 120
hours in an acid solution and loses 150 milligrams. Calculate the corosion rate in mpy and mm/y.
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Home Exercise Problems
Prbs. 1, 4, 8, 10 and 11 of Chapter 1
in “Principles and Prevention of Corrosion”, Denny Jones, Prentice-Hall, 1996.
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Faraday’s Law
Charge is related to mass of material
reacted in an electrochemical reaction:
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Faraday’s Constant
One mole of metal (MW g) contains Avogadro’s number
(6×1023) of metal atoms
Hence each mole of metal will produce n times that many number of electrons
Charge on the electron is 1.6 × 10-19 C (coulomb)
Hence one mole of metal will produce a charge of n × 96500 C
96500 C/equivalent is known as Faraday’s constant
(also in units of J/V⋅equivalent)
Conversions: 1 A (ampere) = 1 C/s, 1 J = 1 C⋅V
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
Faraday’s Law
So, if Q is known, mass loss by corrosion can be determined.
The details of corrosion rate determination by electrochemical techniques will be covered later.
Basics Of Corrosion---------------------------------------------- Next Slide ------------------------------------ Dr. Ramazan Kahraman
References
“Principles and Prevention of Corrosion”, Denny Jones, Prentice-Hall, 1996.
Web Site of Dr. R. A. (Bob) Cottis.
FAQs on Chapter - Basics Of Corrosion, PPT, Semester, Engineering - Electronics and Communication Engineering (ECE)
| 1. What is corrosion and why is it a concern in electronic devices? |  |
| 2. What are the common causes of corrosion in electronic devices? |  |
| 3. How can corrosion in electronic devices be prevented? |  |
| 4. What are the signs of corrosion in electronic devices? |  |
| 5. Can corrosion be repaired in electronic devices? |  |




















