Separation of Substances Class 6 Notes Science Chapter 3

Methods of Separation I-
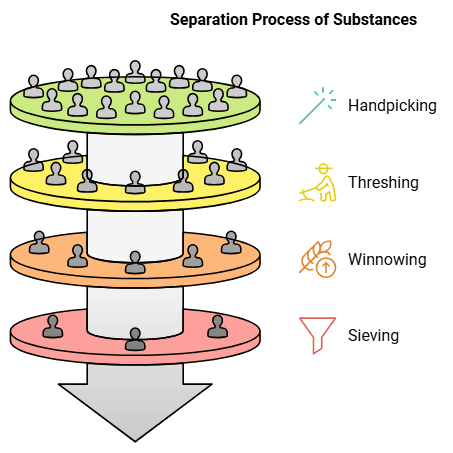
There are various methods for separating substances, such as handpicking, threshing, winnowing and sieving.
Handpicking: It allows the good grains to be separated from the waste and impurities. Handpicking is the basic method for separation of substances. It involves simply picking out substances by hand and separating them from others. The substances being separated may be impurities that have to be thrown away. It could also be that both the substances being separated are useful - such as separating green grapes from black ones.
Threshing: The method of separating the grain from the stalk is called threshing. It is basically the beating of dry stalks to shake off the dried grains. It can be done by hand, by cattle or by machines. Traditionally, threshing was done by hand, but cattle help do the job quickly. Nowadays, threshing machines are also used to separate large quantities of grain quickly.
Winnowing: Even though the large stalks can be separated from the grains by threshing, the grains still have dried husk and chaff, which have to be separated and thrown away before the wheat can be used. This method is called winnowing.
The husk is blown away as it is much lighter than the grain. So, when the grains are gently dropped to the ground, only the wheat grains collect there, while the husk blows away.
Sieving: It is a method of separating substances that are of different sizes. For example, wheat flour has some fine powdered wheat as well as some bigger impurities. When it is put this through a sieve, the fine powder falls through the small holes in the sieve, while the thicker impurities remain as they are too big to pass through these holes. The substances are thus separated.
 Methods of Separation II-
Methods of Separation II-
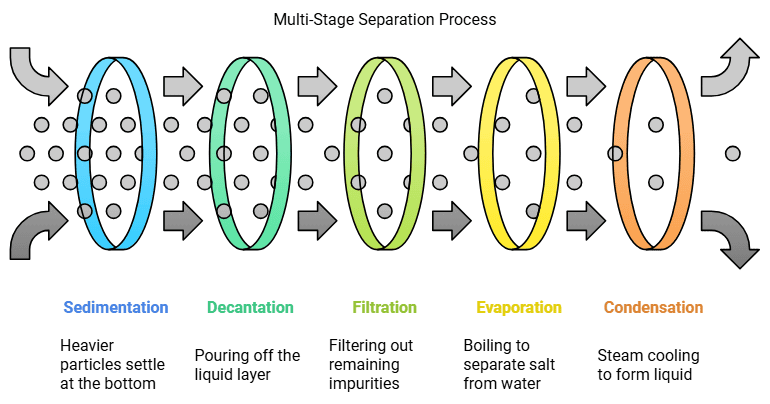
Grains can be separated from dirt by sedimentation and decantation. When water is added to the grains, the dust and dirt dissolve in the water, making it muddy.
Grains can be separated from dirt by sedimentation and decantation. When water is added to the grains, the dust and dirt dissolve in the water, making it muddy. Thus, the dirt and grains are separated. The grains settle at the bottom because they are heavier. This process of the settling of the heavier substance is called sedimentation. Now, the grains can be separated by simply pouring out the water. This process is called decantation.
Oil and water can also be separated by decantation and sedimentation. Water is heavier than oil, so it settles at the bottom if left alone for some time. The oil can then simply be poured out. The water left behind can be further cleaned using a filter paper. While water passes through the fine pores of the filter, dirt will sift out, leaving the water clean. This process of using a filter to separate substances is called filtration.
Salt and water from salty water can be separated by evaporation. We need to boil this water so that its temperature rises and it converts into steam. This process is called evaporation. The steam turns back into water when it comes in contact with a cold metal lid. This process is called condensation. A mixture of sand and salt can be separated by combination of methods. The first method is sedimentation and decantation. This mixture is put in water and left for the sand to settle for some time. Then, we will decant the salty water, which will separate the sand from the mixture. Now the salt can be separated from the water by evaporation. The water will boil away, leaving the salt behind. So, the mixture of the sand, salt and water has been separated successfully using a combination of sedimentation, decantation, evaporation and condensation.
FAQs on Separation of Substances Class 6 Notes Science Chapter 3
| 1. What is the process of separation of substances? |  |
| 2. What are some common methods used for separating mixtures? |  |
| 3. How does filtration work in the separation of substances? |  |
| 4. What is the importance of separating substances in daily life? |  |
| 5. Can you explain the principle behind distillation? |  |






















