Mixture and Solution | Science Class 9 PDF Download
| Table of contents |

|
| Introduction |

|
| Types of Matter |

|
| Solutions |

|
| Alloys |

|
| Suspensions |

|
| Colloidal Solution or Colloids |

|
| Types of Pure Substances |

|
Introduction
- We know that all the matter around us is not pure.
- If we observe some soil (Mitti) and some sugar placed on two different sheets of paper with a magnifying glass, we find soil contains clay particles, some grass particles, and even some dead insects etc. That is soil contains particles of different kinds is called an impure substance (or a mixture).
- Now observe sugar which contains only one kind of particle is called a pure substance.
- We can see that most of the matter around us exist as a mixture of two or more pure components are all mixtures.
Example: Milk, Sea Water, Minerals etc.
Milk: An example of mixture
Types of Matter
On the basis of chemical nature, matter can be classified into two types:
1. Pure Substance
2. Mixtures or Impure Substance
Pure Substances
- A homogeneous material that contains particles of only one kind and has a definite set of properties is called a pure substance.
- A pure substance is a distinct type of matter that cannot be separated into other types of matter by any physical process are pure substance.
Example: Oxygen, Sulphur, Iron etc. - However, if a substance is composed of two or more different kinds of particles combined together in a fixed proportion by weight, then the substance is also regarded as a pure substance.
Example: Sodium chloride is a pure substance because it has a fixed number of sodium and chloride ions, combined together in a fixed proportion by weight.
Similarly, magnesium oxide (MgO), carbon dioxide (CO2), copper sulphate (CuSO4) etc. are pure substances.
 Copper Sulphate
Copper Sulphate
- It does not imply to all homogeneous substances.
Example: A common salt solution in water is a homogeneous solution.
Yet it cannot be called a pure substance, as it is made of two different substances i.e. Salt and Water.
Mixtures or Impure Substance
- When two or more substances (elements, compounds or both) are mixed together in any proportion, such that they do not undergo any chemical change, but retain their individual characteristics the resulting product is called a mixture.
Example: Brass is a mixture of copper and zinc, Crude oil is a mixture of a large number of different hydrocarbons.
 A Mixture
A Mixture
Types of Mixtures
Depending upon the nature of components, a mixture can be divided into two types:
(i) Homogeneous Mixture: A mixture in which different constituents are mixed uniformly, is called a homogeneous mixture. Homogeneous mixtures are also known as solutions. The components of such a mixture cannot be seen even under a microscope.
Example: Salt solution, Copper Sulphate solution, Sugar solution. Similarly alloys such as brass, bronze etc.
(ii) Heterogeneous Mixture: A mixture in which different constituents are not mixed uniformly, is called a heterogeneous mixture. The components of a heterogeneous mixture can be observed with naked eyes or with the help of a microscope.
Example: Sand and iron filings, sand & water etc.
Solutions

- A homogeneous mixture of two or more substances is called a solution. In a solution, there is homogeneity at the particle level.
- Usually, we think of a solution as a liquid that contains either a solid or a liquid or a gas dissolved in it. However, this is not true. We can have a solid solution as in the case of Alloys.
Example: Air is a mixture of gas in gas. Air is a homogeneous mixture of a number of gases. Its two main constituent gases are oxygen (21%) and Nitrogen (78%). - The substances present in a homogeneous solution, are called components of the solution.
A solution basically has two components:
(i) Solvent: The component of a solution that dissolves the other component in itself, is called solvent. A solvent is the larger component of the solution.
Example: A solution of sugar in water is solid in liquid solution. In this solution, sugar is the solute and water is the solvent.
(ii) Solute: The component of the solution which dissolves in the solvent, is called solute. The solute is the smaller component of the solution.
Example: A solution of iodine in alcohol known as 'tincture of iodine', iodine is the solute. Similarly, in carbonated drinks (Soda water), carbon dioxide gas is the solute.
Characteristics of a Solution
- Solution is a homogeneous mixture.
- The size of solute particles in a solution is extremely small. It is less than 1 nm in diameter.
- The particles of a solution cannot be seen even with a microscope.
- The particles of a solution to pass through the filter paper. So, a solution cannot be separated by filtration.
- The solutions are very stable. The particles of solute present in a solution do not separate out on keeping.
- A true solution does not scatter light (because its particles are very small).
Concentration of a Solution
- The concentration of a solution is the amount of solute present in a given quantity of the solution. In other words the mass of the solute in grams, which is present in 100 g of a solution.
- In a solution, the relative proportion of the solute and solvent can be varied. Depending upon the amount of solute present in a solution, it can be called a dilute, concentrated or a saturated solution. Different substances in a given solvent have different solubilities at the same temperature.
- The most common method for expressing the concentration of a solution is called percentage method.
- The concentration of solution refers to the percentage of solute present in the solution.
The percentage of solute can be expressed in terms of:
(i) Concentration of a Solution in Terms of Mass of Solute: If the solution is of a 'solid solute' dissolved in a liquid, then we consider the 'mass percentage of solute' in calculating the concentration of the solution. So, in the case of a solid solute dissolved in a liquid solvent.
Mass by mass percentage of a Solution
where, mass of solution = mass of solute mass of solvent
(ii) Concentration by Mass by Volume Percentage of a Solution.
Mass by volume percentage of a solute in a Solution - Depending upon the unit of the mass and volume, the mass by volume percentage of a solute in a solution can have the following unit, i.e., gram/ml or gram/litre.
Classification of Solution
- Saturated Solution: A solution which at a given temperature dissolves as much solute as it is capable of dissolving, is said to be a saturated solution.
Example: At 30°C, 55 g of common salt dissolves in 100g of water. However, if more of common salt is added to the above solution, it just does not dissolve. In such a situation, the solution of a common salt containing 55 gm of salt in 100 gm of water, is a saturated solution at 30°C.
If a saturated solution at some particular temperature is heated, the solution becomes unsaturated, because of the increase in solubility. If a saturated solution at some higher temperature is cooled, it remains saturated. The excess solute comes out of the solution and deposits itself in the form of crystals. - Unsaturated Solution: When the amount of solute contained in a solution is less than the saturation level, the solution is said to be an unsaturated solution.
Example: At 30°C, if 45 g of common salt is dissolved in 100 g of water, such solution so formed is capable of dissolving more of the common salt, then such a solution is called unsaturated solution. - Supersaturated Solution: A solution which contains more of the solute than required to make a saturated solution is called a supersaturated solution.
Solubility of a Solute
- The amount of solute (in grams), which dissolve in 100 g of water (solvent), at a given temperature, is called solubility of the solute at that temperature.
- Substance (or Solute) Solubility in water (at 20°C):
(i) Copper sulphate, 21 g
(ii) Potassium nitrate, 32 g
(iii) Potassium chloride, 34 g
(iv) Sodium chloride, 36 g
(v) Ammonium chloride, 37 g
(vi) Sugar, 204 g
Factors Affecting Solubility
- Temperature: Solubility increases with temperature. The situation is different for gases. With the increase in temperature, they became less soluble in each other and in water, but more soluble in organic solvents.
- Pressure: For the majority of solid and liquid solutes, pressure does not affect solubility. The solubility of gas is directly proportional to the pressure of this gas.
 |
HOTS Questions: Is Matter Around Us Pure
|
Start Test |
Alloys
- Alloys are homogeneous mixtures of metals and cannot be separated into their components by physical methods. But still, an alloy is considered as a mixture, because it shows the properties of its constituents and can have variable composition.
Example: Brass is a mixture of 30% zinc and 70% copper.
 Brass Keychain
Brass Keychain
Suspensions
- A suspension is a heterogeneous mixture in which the small particles of a solid are spread throughout a liquid without dissolving in it.
- The particles have a tendency to settle down at the bottom of solvent and can be filtered out because their size is bigger than the size of the pores of filter paper.
Example: Chalk-water mixture is a suspension of fine chalk particles in water, Muddy water is a suspension of soil particles in water.Difference between Solution and Suspension
Properties of a Suspension
- A suspension is a heterogeneous mixture.
- The size of solute particles in a suspension is quite large. It is larger than 100 nm in diameter.
- The particles of a suspension can be seen easily.
- A suspension scatters a beam of light passing through it because its particles are quite large.
- The particles of suspension settle down when the suspension is kept undisturbed.
- The process of setting of suspended particles under the action of gravity is called sedimentation. So suspensions are unstable.
Colloidal Solution or Colloids
- A heterogeneous solution in which the particle size is in between 10-7 cm to 10-5 cm such that the solute particles neither dissolve nor settle down in a solvent, is called colloidal solution.
 Comparison among Solution, Colloids, and Suspension
Comparison among Solution, Colloids, and Suspension - The components of colloidal solutions are the dispersed phase and the dispersion medium. The solute-like component of the dispersed particles in a colloidal form of the dispersed phase, and the component in which the dispersed phase is suspended is known as the dispersing medium.
Dispersed Phase: It is the component which is present in small proportion and consists of particles of colloidal dimensions (10-9 m to 10-7 m).Dispersion Medium: It is the component which is present in excess and acts as a medium in which colloidal particles are dispersed.
Properties of Colloids
- The size of particles of a colloid is too small to be individually seen by naked eyes.
- They do not settle down when left undisturbed, that is colloid is quite stable.
- They can not be separated from the mixture by the process of filtration. But, a special technique of separation known as centrifugation can be used to separate the colloidal particles.
- Colloidal solutions are not transparent, but translucent in nature.
- The particles of a colloidal solution scatter light i.e. when a strong beam of light is passed through the colloidal solution, the path of the beam becomes visible.
Common Examples of Colloids
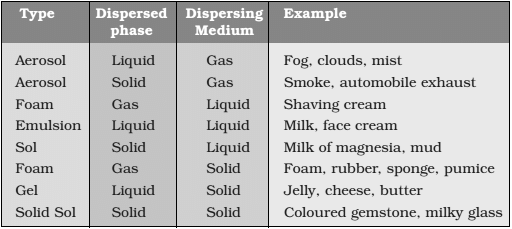
Brownian Movement of Colloids
- The colloidal particles are moving randomly in a zig-zag motion in all directions. This type of zig-zag motion of colloidal particles is called Brownian movement. Brownian movement of colloids
- The Brownian movement is caused by the collision (hitting) of the colloidal particle with the molecules of the dispersion medium.
Tyndall Effect
The phenomenon due to which the path of light becomes visible, due to scattering of light by the colloidal particle is called Tyndall effect.
 Illustration of Tyndall EffectExample: Tyndall effect can also be observed when a fine beam of light enters a room through a small hole. This happens due to the scattering of light by the particles of dust and smoke in the air.
Illustration of Tyndall EffectExample: Tyndall effect can also be observed when a fine beam of light enters a room through a small hole. This happens due to the scattering of light by the particles of dust and smoke in the air.
Tyndall effect can be observed when sunlight passes through the canopy of a dense forest. In the forest, mist contains tiny droplets of water, which act as particles of colloids dispersed in the air.
Difference Between True Solutions and Colloidal Solutions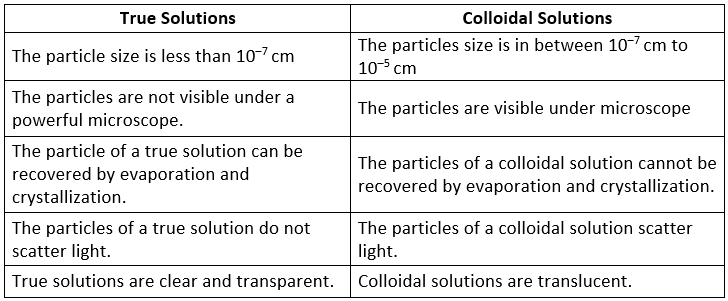
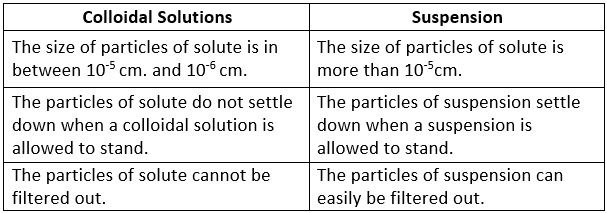
Difference Between Colloidal Solutions and Suspensions
 |
Download the notes
Mixture and Solution
|
Download as PDF |
Types of Pure Substances
 On the basis of their chemical composition, substances can be classified as:
On the basis of their chemical composition, substances can be classified as:
Elements
- Robert Boyle was the first scientist to use the term element is 1661.
- Elements are the basic building block of matter, every substance on the earth made from one or more elements.
- There are 115 elements discovered so far, among these elements, 92 elements occur in nature, whereas 23 have been made in nuclear laboratories, the majority of the elements are solids.
- Eleven elements are in the gaseous state at room temperature.
Two elements are liquid at room temperature: Mercury and Bromine. - Antoine-Laurent Lavoisier (1743-94). A French chemist defined an element as a basic form of matter that cannot be broken down into simpler substances by chemical reactions.
- Elements also can be defined as a substance made up of the atoms with the same atomic number.
- Example: Hydrogen, Oxygen, Nitrogen, Zinc, Mercury etc.
- Elements can be classified as:
(i) Metals
(ii) Non-metals
(iii) Metalloids
(a) Properties of Metals
- They have generally silver grey colour. However, some metal or their alloys have golden yellow colour.
Exception: Copper, which is reddish in colour. - Metals have a lustre, the freshly cut surface has a shine on it.
- They easily conduct heat and electricity.
- They are malleable i.e. they can be beaten into sheets.
- They are sonorous.
- Example: Gold, Silver, Copper, Iron, Sodium, Potassium etc.
Mercury is the only metal that is liquid at room temperature.
(b) Properties of Non-Metals
- They exist in solid, liquid and gaseous state.
- They display variety of colours.
- They are poor conductors of heat and electricity.
- They are generally neither malleable nor ductile.
- They are not sonorous.
- Example: Hydrogen, Oxygen, Iodine, Carbon etc.
(c) Metalloids
- Some elements have intermediate properties of the metals and non-metals. The elements which exhibit the properties of metals, as well as non-metals, are called metalloids.
- Example: Boron, Silicon, Germanium etc.
 Silicon
Silicon
Compounds
- A pure substance, which is composed of two or more elements, combined chemically in a definite ratio, such that it can be broken into elements only by chemical means, is called a compound.
- The two or more elements present in a compound, are called constituents or components of the compound.
Example: Water is a compound of hydrogen and oxygen, combine together in the ratio of 1:8 by weight. The water can be broken into its constituents only by electrochemical method i.e. by passing an electric current through it. - The compounds can be further classified as:
(i) Acids
(ii) Bases
(iii) Salts
The product formed by mixing 1g of sulphur powder and 2g of iron filings/turnings called a mixture.
- The constituents of the product i.e. iron filings and yellow particles of sulphur can be seen with naked eye, which is the property of a mixture.
- The iron fillings can be separated by dissolving the mixture in carbon disulphide, sulphur dissolves, but not the iron. As the constituents can be separated by physical means, therefore, the product is a mixture.
- The constituents iron and sulphur are not evenly spread. At some places, iron filings are more than sulphur.
- No energy is absorbed when the sulphur powder is mixed with iron filings.
- The particles of iron and sulphur retain their individual chemical and physical properties.
The product formed on heating 1g of sulphur powder and 2g of iron turnings is called a compound.
- The product formed is iron sulphide the yellow particles of sulphur and filings of iron are no longer visible.
- The iron or sulphur cannot be separated from the iron sulphide by any physical means.
- The composition of iron sulphide is same throughout.
- Heat energy is evolved when the iron reacts with sulphur. The product continues glowing with red dull colour, even when the heating is stopped.
- The properties of the product (iron sulphide) are entirely different from the properties of iron and sulphur.
Difference Between Mixtures and Compounds
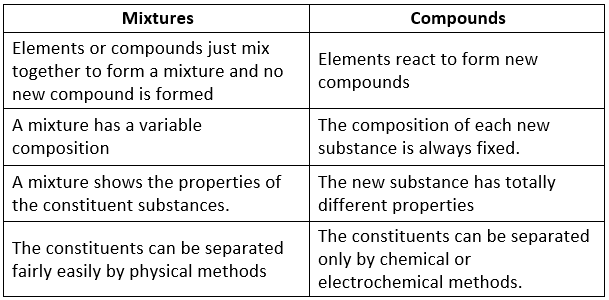
|
84 videos|394 docs|61 tests
|
FAQs on Mixture and Solution - Science Class 9
| 1. What are the different types of matter? |  |
| 2. What are solutions? |  |
| 3. What are alloys? |  |
| 4. What are suspensions? |  |
| 5. What are colloidal solutions or colloids? |  |



























