Equilibrium in Physical Processes | Chemistry Class 11 - NEET PDF Download
What is Equilibrium?
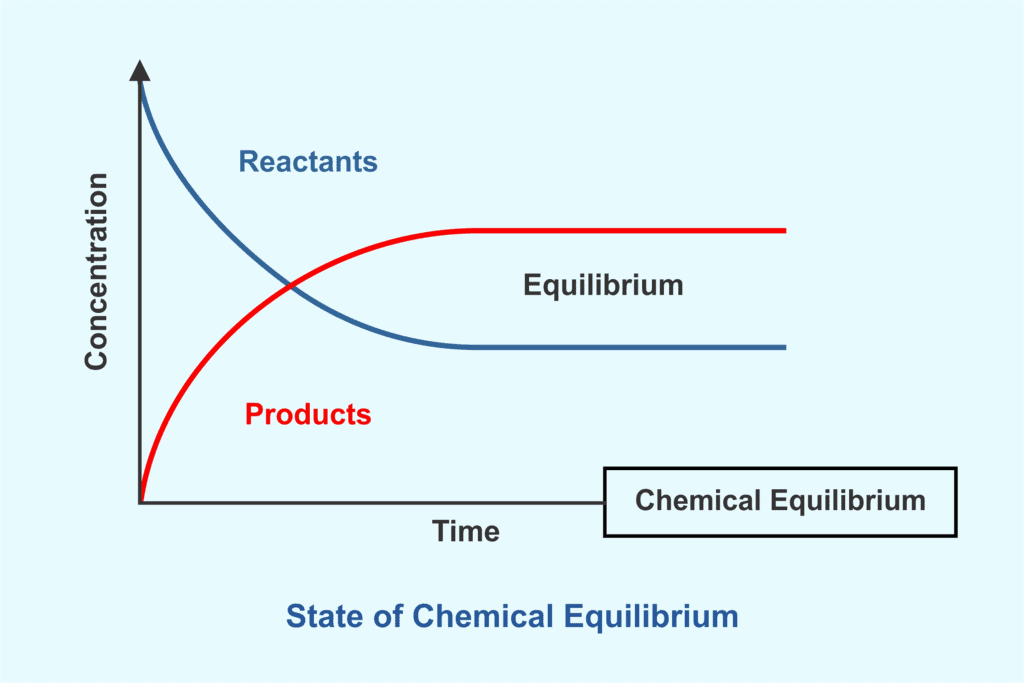
Equilibrium represents a state in a process where measurable properties such as pressure, temperature, and concentration remain unchanged over time. It occurs when opposing forces balance each other, leading to a dynamic but stable condition.
Common Terms in Equilibrium
1. Equilibrium Mixture
An equilibrium mixture is a system in which both reactants and products are present in constant concentrations. This occurs in a reversible reaction, where the forward and backward reactions take place at the same rate.
2. Dynamic Equilibrium
When two opposing processes occur simultaneously and independently, forming a stable interchange between two states, the system is in dynamic equilibrium. It is represented by a double-sided arrow:
3. Chemical Equilibrium
Chemical equilibrium refers to a state in which the rate of the forward reaction equals the rate of the reverse reaction, ensuring no further change in reactant or product concentrations.
4. Ionic Equilibrium
Ionic equilibrium occurs when an ionic compound dissociates into its ions in a polar solvent. The equilibrium exists between the ions and the undissociated solute in the solution.
Example:
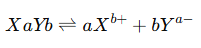
Equilibrium in Physical Processes (Physical Equilibrium)
Physical equilibrium is defined as the equilibrium which develops between different phases or physical properties. In these processes, there is no change in chemical composition.
Types of Physical Equilibria:
- Phase Equilibrium – Balance between different states of matter.
- Solute-Solid Equilibrium – Equilibrium between a solid and its solution.
- Gas-Liquid Equilibrium – Balance between gases dissolved in a liquid and gaseous form.
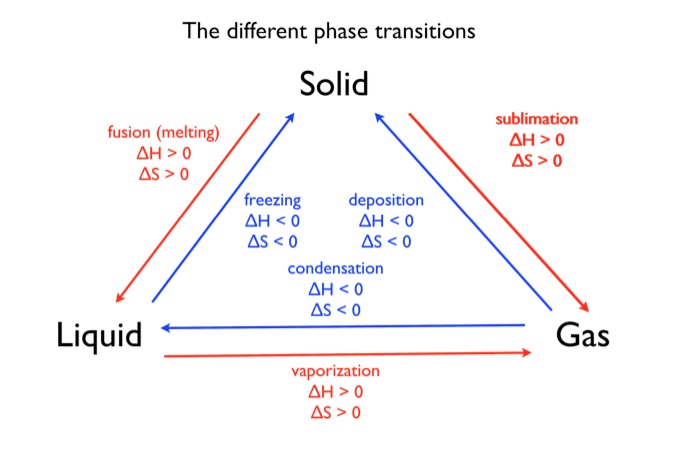
Let’s try and understand equilibrium in physical processes in more detail. Substances undergo different phase transformation processes such as:
- solid ⇌ liquid
- liquid ⇌ gas
- solid ⇌ gas
Solid-Liquid Equilibrium
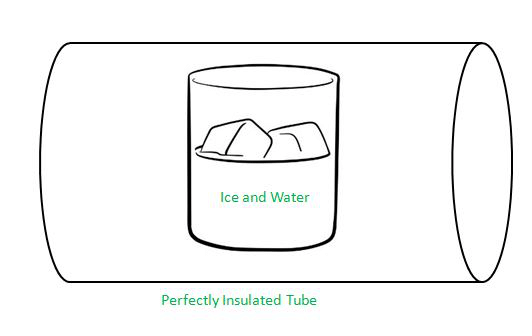 Solid Liquid Equilibrium
Solid Liquid Equilibrium
What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask at a temperature of 273K and atmospheric pressure?
We see that the mass of ice and water do not change and that the temperature remains constant, indicating a state of equilibrium.
However, the equilibrium is not static because there is intense activity at the boundary between ice and water. Some ice molecules escape into liquid water and some molecules of water collide with ice and adhere to it. Despite this exchange, there is no change in the mass of ice and water. This is because the rates of transfer of ice molecules to water and the reverse process are equal at 273K and atmospheric pressure.
It is evident that ice and water are in equilibrium only at a particular pressure and temperature. Therefore,
For any pure substance at atmospheric pressure, the temperature at which the solid and liquid phases are at equilibrium is called the normal melting point or normal freezing point of the substance.
The system of ice and water is in dynamic equilibrium and we can conclude the following:
- Both opposing processes occur at the same time.
- The two processes occur at the same rate such that the amount of ice and water remains constant.
Liquid - Vapour Equilibrium
 Liquid Vapour Equilibrium
Liquid Vapour Equilibrium
To understand this concept, let’s perform the following experiment.
Experiment:
- Place a drying agent like anhydrous calcium chloride for a few hours in a transparent box with a U-tube containing mercury i.e. manometer. This will soak up all the moisture in the box.
- Remove the drying agent by tilting the box to one side and quickly place a petri dish containing water.
Observations:
- The mercury in the manometer rises slowly and then attains a constant value. This is because the pressure inside the manometer increases due to the addition of water molecules into the gaseous phase.
- Initially, there is no water vapour in the box. As the water in the petri dish evaporates, the volume of water in the petri dish decreases and the pressure in the box increases.
- The rate of increase in pressure decreases with time because of the condensation of vapour into water.
- Finally, it reaches an equilibrium where there is no net evaporation or condensation.
Conclusion:
- Equilibrium is reached when the rate of evaporation = rate of condensation
H2O(l) ⇌ H2O (vap) - At equilibrium, the pressure that the water molecules exert at a given temperature remains constant and is called the equilibrium vapour pressure of water. The vapour pressure of water increases with temperature.
Boiling Point
At the same temperature, different liquids have different equilibrium vapour pressures. The liquid with a higher vapour pressure is more volatile and has a lower boiling point.
Solid - Vapor Equilibrium
Have you ever observed what happens to solid iodine placed in a closed jar? The jar gets filled up with violet coloured vapour and the colour intensity increases with time! The intensity of the colour becomes constant after a certain time i.e. equilibrium is reached. In this way, solid iodine sublimes to give iodine vapour while the vapour condenses to form solid iodine. The equilibrium in this process is given as
I2(solid) ⇌ I2(vapour)
Other substances that show this kind of equilibrium are:
- Camphor (solid) ⇌ Camphor (vapour)
- NH4Cl (solid) ⇌ NH4Cl (vapour)
Equilibrium Involving Dissolution of Solid or Gases in Liquids
 |
Download the notes
Equilibrium in Physical Processes
|
Download as PDF |
Solids In Liquids
What happens when you make a thick sugar solution by dissolving sugar at a high temperature, then allow it to cool at room temperature?
Yes, the sugar crystals separate out.
In this case, the thick sugar solution is a saturated solution because no more solute i.e. sugar can be dissolved in it at a given temperature. The concentration of solute in a saturated solution depends on the temperature. A dynamic equilibrium exists between the solute molecules in the solid-state and in solution in a saturated solution.
Sugar (solution) ⇌ Sugar (solid)
Also, the rate of dissolution of sugar = the rate of crystallization of sugar.
Let’s understand this further, using an example.
What happens when you add radioactive sugar to a saturated solution of non-radioactive sugar?
- You will see radioactivity both in the solution and solid sugar after some time. Initially, there are no radioactive sugar molecules in the solution.
- But, due to the dynamic nature of equilibrium, there is an exchange between the radioactive and non-radioactive sugar molecules from the two phases.
- Thus, the ratio of radioactive to non-radioactive sugar molecules in the solution increases till it reaches a constant value.
Gases In Liquids
Why do we see fizz and hear a sound when we open soda bottles?
This happens because some of the CO2 dissolved in it fizzes out rapidly due to the difference in solubility of CO2 at different pressures. The equilibrium between the CO2 molecules in the gaseous state and those dissolved in liquid under pressure is given as:
CO2(gas) ⇌ CO2(in solution)
 Gases in Liquids
Gases in Liquids
This equilibrium is governed by Henry’s law.
It states that the mass of a gas dissolved in a given mass of a solvent at any temperature is proportional to the pressure of the gas above the solvent. This amount decreases with increase in temperature.
The soda bottle is sealed under the pressure of the gas where its solubility in water is high. When the bottle is opened, some of the CO2 escapes trying to reach a new equilibrium or its partial pressure in the atmosphere. This is why soda water turns flat when the bottle is left open for too long.
Some important points to remember are as follows:
- For solid ⇌ liquid equilibrium, there exists a specific temperature (melting point) at which both phases can coexist under 1 atm pressure. Without heat exchange with the surroundings, the mass of the phases remains constant.
- For liquid ⇌ vapor equilibrium, the vapor pressure remains constant at a given temperature.
- The solubility of solids in liquids remains constant at a given temperature.
- The concentration of a gas dissolved in a liquid is directly proportional to the pressure (concentration) of the gas above the liquid.
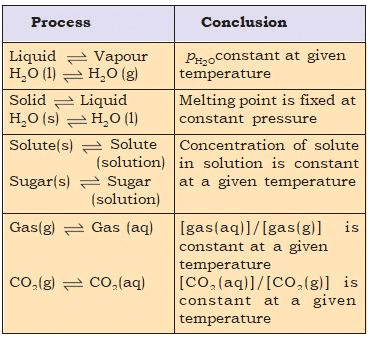
Some Features of Physical Equilibria
General Characteristics Of Equilibrium In Physical Processes
The following characteristics are common to the state of equilibrium in physical processes:
- At a given temperature, equilibrium in physical processes is achieved only in a closed system.
- The opposing processes occur at the same rate and there exists a dynamic but stable condition during equilibrium in physical processes.
- All properties of the system that are measurable remain constant.
- Equilibrium in physical processes is characterized by a constant value of one of its parameters at a given temperature.
- The extent to which a physical process has progressed before reaching equilibrium is indicated by the magnitude of the abovementioned parameter at any stage.
|
127 videos|245 docs|87 tests
|
FAQs on Equilibrium in Physical Processes - Chemistry Class 11 - NEET
| 1. What is physical equilibrium? |  |
| 2. What are some common terms used in the context of equilibrium in physical processes? |  |
| 3. How is equilibrium involving the dissolution of solids or gases in liquids achieved? |  |
| 4. What are some general characteristics of equilibrium in physical processes? |  |
| 5. How does equilibrium in physical processes relate to Le Chatelier's principle? |  |
























