Minerals, Ores & Concentration of Ores | Chemistry for JEE Main & Advanced PDF Download
Minerals, Ores and Concentration of Ores:
Minerals: Naturally occurring chemical substance in which metal exist either in its free state or in combined state is called mineral.
Ore: Mineral from which metal can be conventionally and economically extracted is called ore & impurities associated with it is called gangue or matrix.
Types of Ores:
| Sulphide Ores: | |
| Galena : PbS, | Cinnabar : HgS, |
| Zinc blend : Zns, | Chalcopyrite : CuFeS2 |
| Copper glance : Cu2S | Fool's Gold : FeS2 |
 Fig: Galena
Fig: Galena
| Oxide Ores: | |
| Bauxite : Al2O3. 2H2O | Haematite : Fe2O3 |
| Limonite : Fe2O3. 3H2O | Tin stone or Cassiterite : SnO2 |
Carbonate Ores: Siderite : FeCO3 Calamine ZnCO3
Malacite: Cu(OH)2CuCO3 Dolomite CaCO3. MgCO3. 2H2O
lime stone: CaCO3 Fig: Lime stone
Fig: Lime stone
| Sulphate Ores: | |
| Gypsom : CaSO4.2H2O | Anylesite PbSO4 |
| Glauber's salt : Na2SO4. 10 H2O | Mohr's salt : FeSO4. (NH4)2SO4. 6H2O |
| Halide Ores: | |
| Rock salt : NaCl | Cryolite : Na3AlF6 |
| Fluorspar : CaF2 | Carnallite : KCl. MgCl2. 6H2O |
Nitrate Ores: Chile Saltpeter : NaNO3
Indian Salt petre : KNO3
Native Ores: Those metals which are chemically less reactive. They occur in the earth crust in form of free state (lumbs)
e.g : Cu, Ag, Au, Hg, Pd, Pt, Bi.
General principles and processes involved in the extraction of metal from its ore:
The extraction of metal from its ore is completed in five steps:
Step I: Pulverization: The crushing of ore to powdered state is called pulverization.
Step II: concentration or Dressing or Beneficiation of ore
Step III: Conversion of Concentrated ore into oxide form
step IV: Reduction of oxide to the metal
Step V: Purification or refining of crude metal:
Step I: Pulverization: The crushing of ore to powdered state is called pulverization
This process in stamp mill or ball mill
Step II: Concentration or Dressing or beneficiation of Ore
(a) By Gravity separation: Ore particles are heavier than the gangue particles. This is used for the separation of most of the gangue particles : Fig: Gravity separation⇒ By Wilfley Table Method
Fig: Gravity separation⇒ By Wilfley Table Method
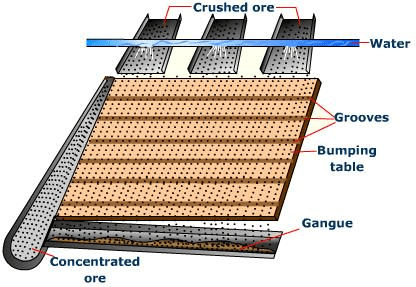 Fig: Wilfey Table method
Fig: Wilfey Table method
⇒ By Hydraulic Classifier
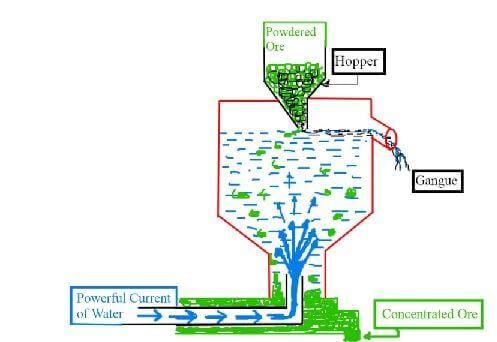 Fig: Hydraulic classifier(b) By Magnetic separator :
Fig: Hydraulic classifier(b) By Magnetic separator :

Cassiterite or Tinstone contains impurities of wulframite or wulframates of Fe & Mn.
⇒ Tin stone : SnO2 → Diamagnetic
⇒ Wulframites or wulframates of
Fe & Mn : FeWO4, MnWO4 ⇒ Paramagnetic.
Ulframates of Fe & Mn from Tin stone by magnetic separator.
(c) By Froth Floatation Process: This method is used for the concentration of sulphide ores.
It is based on the concept that the sulphide ores are preferentially wetted by pine oil, camphor oil while gangue particles are preferentially by water.
This is based on the physical phenomenon of adsorption.
 Frother: Pine oil, Camphor oil
Frother: Pine oil, Camphor oil
Froth Stabilizers: They reduce surface tension of water e.g. cresols, amines.
Collector: Sodium or Potassium xanthates. It combines with sulphide ore & makes them water replent so that its affinity towards pine oil increases (Adsorption tendency increases)
KOH + EtOH → EtO- + K + H2O

Depressant: KCN or NaCN
ZnS is found to be an impurity with the lead sulphide. Therefore to separate out PbS from ZnS depressant KCN or NaCN added.
PbS NaCN → No complex formation due to very law Ksp of PbS.
ZnS 4NaCN → 4Na [Zn (CN)4]2- S- -
(water soluble)
Thus, ZnS becomes water soluble & it remains with gangue while PbS comes out with the froth.
Activator: CuSO4
From galena (PbS.ZnS) ZnS is removed
ZnS + 4NaCN  4 Na + [Zn(CN)4]2-
4 Na + [Zn(CN)4]2-  [Cu(CN)4]3- + ZnS ¯ + S- -
[Cu(CN)4]3- + ZnS ¯ + S- -
(water soluble)
(more stable)
& ZnS is taken out by froth floatation second time.
(ii) Chemical Method of Concentration :
Leaching : It involves dissolution of metallic ore in a suitable reagent in which metallic ore is soluble and impurities are insoluble.
Leaching of alumina from bauxite :
Al2O3(s) 2NaOH(aq) 3H2O(I) → 2Na[Al(OH)4](aq)
Having F2O3 as important leachant.
The aluminate in solution is acidified by adding acid and hydrated Al2O3 is precipitated.
2Na[Al(OH)4](aq) + H+ (aq)  Al(OH)3 ¯ + H2O + Na+ (aq).
Al(OH)3 ¯ + H2O + Na+ (aq).
(white ppt)
Hydrated alumina is filtered, dried and heated to give back pure Al2O3 :
Al(OH)3¯  Al2O3(s) + 3H2O(g)
Al2O3(s) + 3H2O(g)
white (pure)
Other examples :
In the metallurgy of silver and gold, the respective metal/ore is leached with very dilute solution of NaCN or KCN in the presence of air (for O2) from which the metal is obtained by displacement reaction.
4M(s) +8CN-(aq) +2H2O(aq) +O2(g) → 4[M(CN)2]- (aq) +4OH-(aq)
(M = Ag Or Au)
2[M(CN)2]- (aq) +Zn(s) → [Zn(CN)4]2-(aq) +2M(s)¯
Step III : Conversion of Concentrated ore into oxide form :
It is done either by calcination or by roasting.
(i) Calcination :
Calcination is carried out for carbonate, hydrated metal oxide & metal hydroxide ores.
It is carried out in the absence of air i.e., heating in absence of air.

Due to calcination ore becomes porous.
Volatile organic impurities get evaporated


(ii) Roasting : In the presence of air the sulphide are heated in free supply of air below m.p. Impurities of sulphur, phosphorus, arsenic & antimony are converted into their corresponding volatile oxide & thus get removed.
Moisture & Water of crystallization are also removed.

Step IV : Reduction of oxide to the metal :
(b) Reduction of Metal oxide / conc. ore into free metal .
This can be carried out
(i) chemical reduction
(ii) By self reduction or auto reduction or Air Reduction
(iii) Metal - displacement method
(iv) By electrolytic Reduction
(v) By amulgamation.
(i) & (ii) method are collectively known as Pyrometallurgy
e.g. Sn, Pb, Fe, Hg, Cu, B, Zn, (Based on Ellinghum diagram)
(iii) step is called hydrometallurgy — Cu, Ag, Au are extracted
(iv) step is called Electrometallurgy, Alkali, Alkaline earth metals & Al & base electrolysis
(v) is used for Ag & Au
(i) Chemical Reduction :
1. Smelting i.e., carbon Reduction - Reduction of metal oxide by coke, coal & RO
Reduction of the metal oxide usually involves heating it with some other Substance acting as a reducing agent, e.g., C or CO or even another metal. The reducing agent (e.g., carbon) combines with the oxygen of the metal oxide.
MxOy + yC → xM y + CO
Some metal oxides get reduced easily while others are very difficult to be reduced. To understand the variation in the temperature requirement for thermal reductions and to predict which element will suit as the reducing agent for a given metal oxide (MxOy), Gibbs energy interpretations are done, which is explained by ellingham diagram.
ΔG = ΔH - TΔS
If ΔH is greater than zero then reduction will be feasible on increasing temprature i.e., |TΔS| > |ΔH|
Ellingham diagram:
M(s) +  O2(g)
O2(g)  M2Ox(s)
M2Ox(s)
ΔG = ΔH - TΔS
Therefore, For forward rxn ΔS < 0
 Fig: Ellingham diagram
Fig: Ellingham diagram
(Ellingham diagram for formation of M2Ox)
Ellingham diagram is a plot of formation of an element oxide between ΔG & ΔT
Ex. Which of the following statements are true :
A → Mg(s) +  O2(g)
O2(g)  MgO(s)
MgO(s)
B → Mg(l) +  O2(g)
O2(g)  MgO(s)
MgO(s)
C → Mg(g) +  O2(g)
O2(g)  MgO(s)
MgO(s)

I : Below 1350° Mg can reduce Al2O3
II : Above 1350° C Mg Will reduce Al2O3
III : Below 1350° Al can reduce MgO
IV : Above 1350° Al can reduce MgO
V : At 1350° C there is no change in free energy i.e., ΔG = 0
Sol.  ΔG < 0
ΔG < 0
(Its ΔG high) (Its ΔG less)
Al2O3 + 3 Mg  3 MgO + 2Al
3 MgO + 2Al
At 1350° C both reactions have same G Therefore,ΔG = 0
To carry out smelting below 800°C, CO is used as reducing agent while above 800°C, smelting is carried out by coke.
2C(s) + O2(g) → 2CO (g) + ΔH = -221.0 kJ/mole
DS = 179.4 J kJ/mol
C(s) + O2(g) → CO2(g) + ΔH = - 393.5 kJ/mol
ΔS = 2.89 JK-1 mole-1

Aluminium can be extracted from Alumina by carbon reduction but the method is highly uneconomical because -
(i) As the smelting occurs above 200°C hence a part of the aluminium will go into vapour phase (M.P. = 2520°C)
(ii) At this high temperature the liberated Al will combine with the carbon & aluminium carbide will be formed.
(iii) ΔHfor of alumina is high - ve value
Therefore,It is thermodynamically more stable & reduction is more difficult
To extract metal from sulphide ore is carried out by firstly roasting it into metal oxide & followed by its smelting. Metal sulphide or sulphide ore is not directly smelted to metal.
2PbS + C  2Pb + CS2 (Thermodynamically Not feasible)
2Pb + CS2 (Thermodynamically Not feasible)
Pbs +  O2
O2  PbO + SO2
PbO + SO2
PbO + C  Pb CO Thermodynamically feasible
Pb CO Thermodynamically feasible
ΔGf of PbS = -21.9 kcal/mol
ΔGf of CS2 = 17.15 kcal/mol
ΔGf of PbO = -45.1 kcal/mol
ΔGf of SO2 = -71.7 kcal/mol
ΔGf of CO = - 32.8 kcal/mol
Flux : Additional substances which are used during metal extration to remove acidic or basic impurity are called flux depending upon nature of impurity flux are of two types.
(i) Basic Flux : It is used to remove acidic impurity eg : CaO, MgO, CaCO3, MgCO3 FeCO3 etc.
(ii)Acidic Flux : It is used to remove basic impurity eg : SiO2, B2O3, P2O5, Na2B4O7. (Borax)
Smelting : Phenomenon of slag formation by combining flux with impurity is called smelting.
Flux + Impurity → Slag (Smelting)
(Basic or acidic)
Properties of slag :
(i) Slag has low melting point than metal.
(ii) Slag is lighter than metal therefore it floats over the molten metal and prevents further oxidation of molten metal by air.
(iii) Slag immiscible with molten metal therefore it can be easily separated from molten metal.
(b) Gold Schmidt Thermite Reduction :
Thermite : Al powder
Cr2O3 + 2Al  2Cr + Al2O3
2Cr + Al2O3
(DGf = -540 kJ/mole) (DG3 = -827 kJ/mole)
B2O3 + 2Al  2B + Al2O3
2B + Al2O3
2Mn3O4 + 8Al  9Mn + 4 Al2O3
9Mn + 4 Al2O3
Fe2O3 + 2Al  2Fe + Al2O3
2Fe + Al2O3
This method is used for reduction of those metal oxides which are highly stable if they are reduced by coke it will occur at very high temperature & at this high temperature the liberated metal will combine with the coke & carbide will be formed hence Al powder i.e., thermite is used
(c) Reduction by Hydrogen :
Because of inflammable nature of hydrogen its use as a reducing agent is very restricted.
Cu2O + H2  2Cu + H2O
2Cu + H2O
MOO3 + 3H2  M0 + 3H2O
M0 + 3H2O
BCl3 + 3/2H  B + 3HCl
B + 3HCl
Reduction by other metals :
SiCl4 + 2Mg  2MgCl2 Si
2MgCl2 Si
Kroll process used for extraction of Ti & Zr
TiCl4 + 2Mg Ti 2MgCl2
ZrCl4 2Mg  Zr 2MgCl2
Zr 2MgCl2
I.M.I Process (Imperial Metal Industries)
TiCl4 4Na  Ti 4NaCl
Ti 4NaCl
(ii) By Self reduction or Auto reduction or Air Reduction :
This method is used for extraction of copper, lead, mercury i.e., it is used for the extraction of metal from their sulphide ores.
In this method the sulphide ore is roasted in free supply of air to its metal oxide & then air supply is cut off followed by heating by increasing temperature & metal is extracted by self reduction.
PbS + 
 PbO + SO2
PbO + SO2
PbS + 2O2  PbSO4
PbSO4
Now air supply is cut off followed by heating
PbS(s) + 2PbO(s)  3Pb(l) + SO2
3Pb(l) + SO2
Self reduction is responsible for acid rain than roasting because SO2 dissolves in air, (3927cc CO2 in 1000cc of H2O)
(iii) By Metal Displacement Method or By Hydrometallurgy:


In this method the concentrated ore is treated/ leached with specific chemical reagent that converts the ore into water soluble salt. Now, on adding more electropositive metal into the aqueous salt solution the metal (less electropositive) is displaced
e.g.

Iron is found to be an impurity in the copper ores hence if Zn is added to extract copper, iron will also be displaced along with copper & that is why iron is used.
Both metals which extracted & by which we extracted are water insoluble
(iv) Electro Metallurgy: The metal is extracted by passing electricity into its fused salt or in aqueous solution.
Extraction of sodium:
⇒ By electrolysis of Aq. NaCl solution :
NaCl(s) x H2O  Na (aq) Cl-(aq)
Na (aq) Cl-(aq)
H2O  H OH-
H OH-
On passing electricity
At cathode : 2H 2e-  H2
H2
ΔG = - n FE°
Therefore,Na does not discharge at cathode
At anode : 2Cl-  Cl2 2e-
Cl2 2e-

In sol : Na OH-  NaOH
NaOH
⇒ By electrolysis of fused NaCl :
On Passing electricity
At cathode : 2Na + 2e-  Na
Na
At Anode : 2Cl-  Cl2 + 2e-
Cl2 + 2e-
In sol. Na + OH-  NaOH
NaOH
Electrochemical Principles of Metallurgy:
We have seen how principles of thermodynamics are applied to pyrometallurgy. Similar principles are effective in the reductions of metal ions in solution or molten state. Here they are reduced by electrolysis or by adding some reducing element.
In the reduction of molten metal salt, electrolysis is done. Such methods are based on electrochemical principles which could be understood through the equation,
ΔG° = -nE°F ..................... (16)
Here n is the number of electrons and E° is the electrode potential of the redox couple formed in the system. More reactive metals have large negative values of the electrode potential. So their reduction is difficult. If the difference of two E° values corresponds to a positive E° and consequently negative DG° in equation (16), then the less reactive metal will come out of the solution and the more reactive metal will go to the solution, e.g.,
Cu2+ (aq) + Fe(s) → Cu(s) + Fe2+ (aq)
In simple electrolysis, the Mn ions are discharged at negative electrodes (cathodes) and deposited there. Precautions are taken considering the reactivity of the metal produced and suitable materials are used as electrodes. Sometimes a flux is added for making the molten mass more conducting.
|
352 videos|596 docs|309 tests
|
FAQs on Minerals, Ores & Concentration of Ores - Chemistry for JEE Main & Advanced
| 1. What are minerals and why are they important in our daily lives? |  |
| 2. What is an ore and how is it different from a mineral? |  |
| 3. How are ores concentrated? |  |
| 4. What factors determine the choice of concentration method for a particular ore? |  |
| 5. Can all ores be economically concentrated? |  |
|
352 videos|596 docs|309 tests
|

|
Explore Courses for JEE exam
|

|


















