Conformational Isomerism | Chemistry Class 11 - NEET PDF Download
| Table of contents |

|
| Conformers |

|
| Conformational Isomerism in alkanes |

|
| Newman Projection of Ethane |

|
| Rotational Barrier & Torsional Strain |

|
| Stability of cycloalkanes: |

|
| Conformation of Cyclohexane |

|
Conformers
Different spatial arrangements of the atoms that result from restricted rotation about a single bond are called conformations. Different conformations are also called conformational isomers or conformers.
Conformational Isomerism in alkanes
- When an ethane molecule rotates about its carbon-carbon single bond, two extreme conformations can result the staggered conformation and the eclipsed conformation.
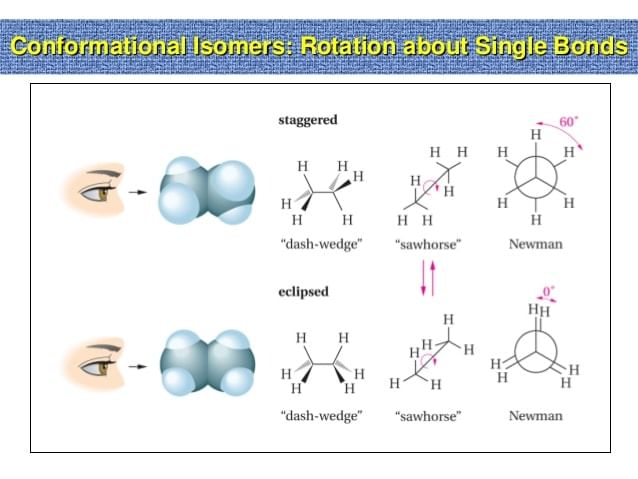
- An infinite number of conformations between these two extreme conformations is also possible. There are several ways to represent on paper the three-dimensional conformation that occurs as a result of rotation about a single bond.
- Wedge-and-dash structures, Sawhorse projections and Newmann projections are all commonly used methods.
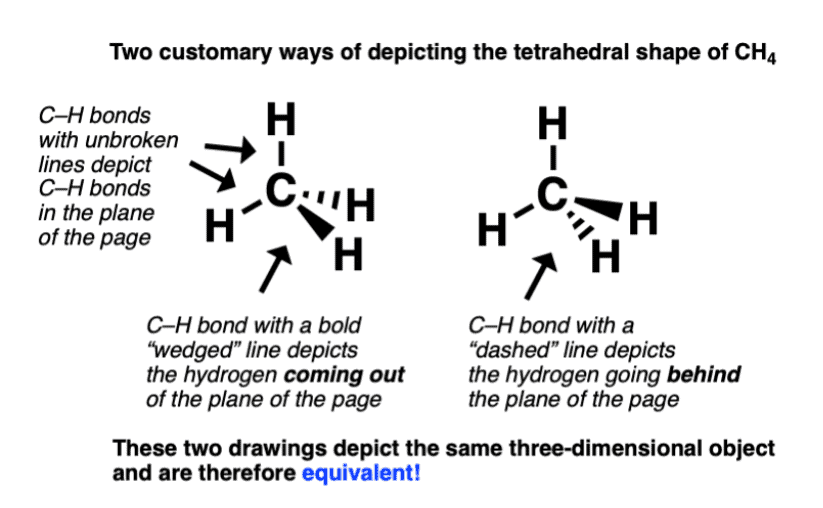
- But here we will use only Newmann projections. In a Newmann projection, you will look down the length of a particular carbon-carbon single bond.
- The carbon that is in the front, is represented by the point at which three bonds intersect and the carbon that is in the back is represented by a circle.
- The three lines emanating from each of the carbons represent the carbon's other three bonds.
Newman Projection of Ethane
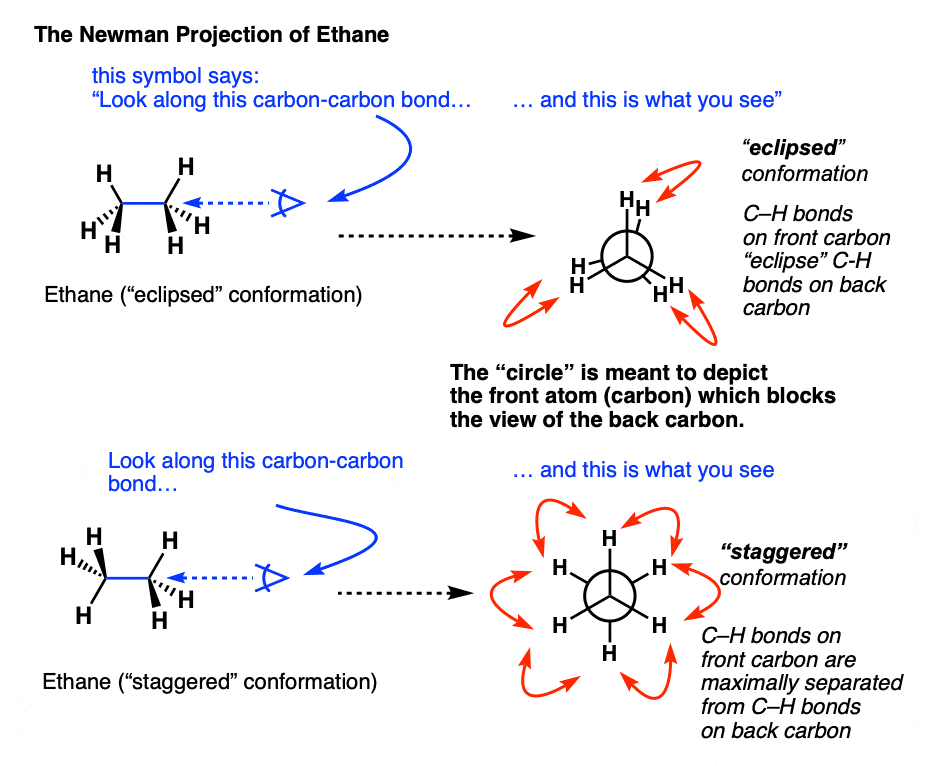
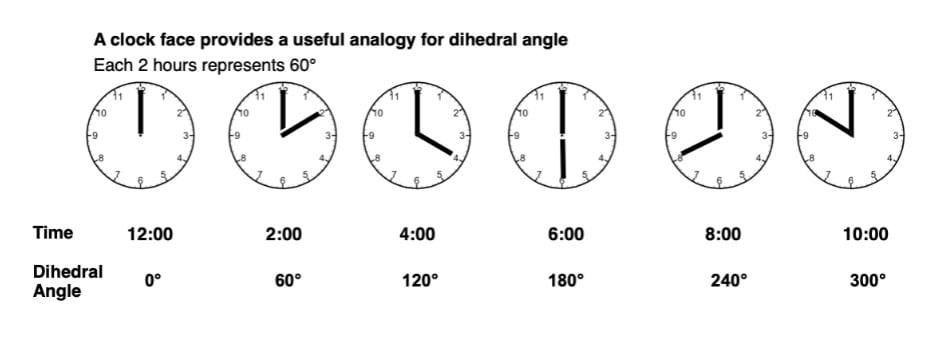
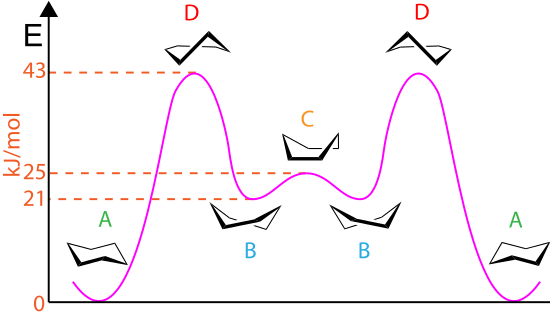
Staggered conformations : A conformation with a 60º dihedral angle is known as staggered conformation.
Eclipsed conformation : A conformation with a 0º dihedral angle is known as eclipsed conformation.
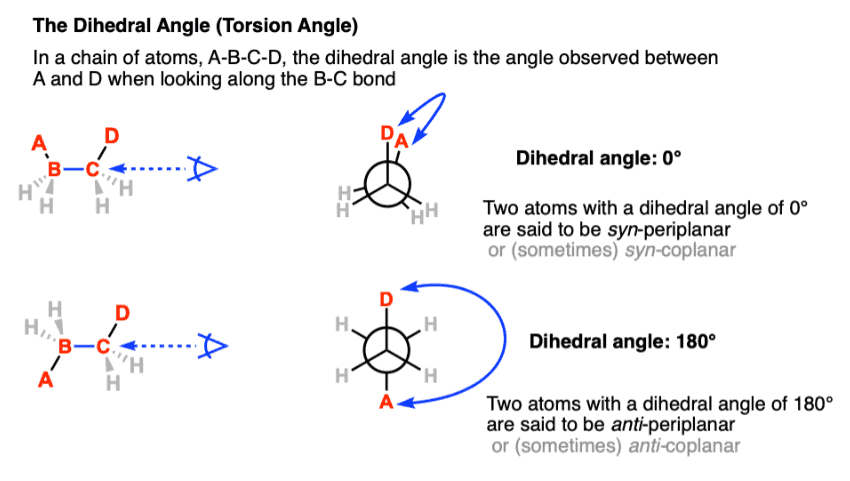
- The electrons in a carbon-hydrogen bond will repel the electrons in another carbon-hydrogen bond if the bonds get too close to each other.
- The staggered conformation, therefore, is the most stable conformation because the carbon-hydrogen bond are as far away from each other as possible.
- The eclipsed conformation is the least stable conformation, because in no other conformation are the carbon-hydrogen bond closer to one another.
- In staggered conformation the distance between the hydrogen nuclei is 2.55 Å. but, they are only 2.29 Å apart in the eclipsed conformation.
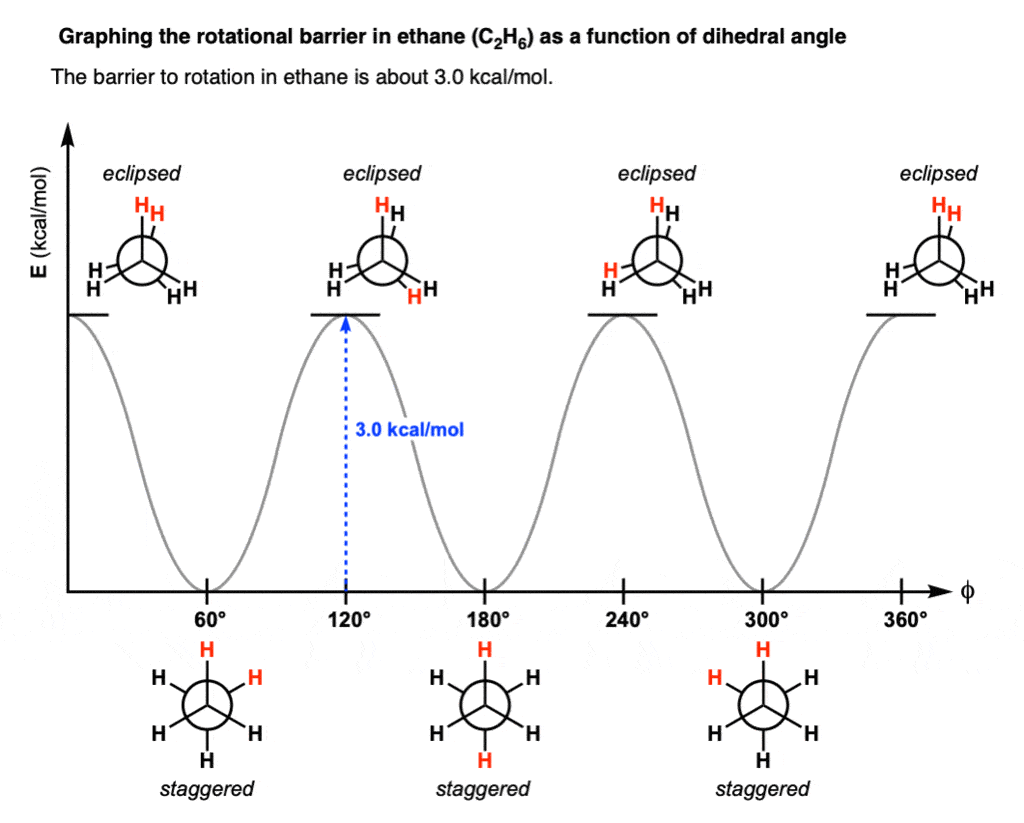
Rotational Barrier & Torsional Strain
- The rotational barrier in ethane is 2.9 Kcal/mole.
- This rotational barrier can be described in terms of the change in potential energy of the molecule as a function of the change in dihedral angle.
- The extra energy of the eclipsed conformation is called torsional strain.
- Torsional strain is the name given to the repulsion felt by bonding electrons of one substituent as they pass close the bonding electrons of another substituent.
- The energy barrier between staggered and eclipsed conformation in ethane molecule is 2.9 Kcal/mole (12 kJ/mole).
- This barrier is more than RT ( » 0.6 Kcal/mole) at room temperature (energy for free rotation) and less than 16-20 Kcal/mole (energy barrier for frozen rotation). Hence the rotation about carbon-carbon single bond is neither completely free nor frozen but only restricted.
Note : (i) For free rotation energy barrier is 0.6 Kcal/mole.
(ii) For restricted rotation energy barrier is in between > 0.06 and < 16 Kcal/mole.
(iii) For frozen rotation energy barrier is 16 Kcal/mole.
Similarly, propane has also two conformations.
In this case, out of six substituents on two C's (carbon -1 and carbon-2 ) five, are hydrogens and one is CH3 group.
Butane has three carbon-carbon single bonds and the molecule can rotate about each of them.
If rotation will be about C - 2 and C -3 bond then conformation will be symmetrical.
For conformational analysis, treat butane as the derivative of ethane. Out of six substituents, four are hydrogens and two are methyl groups. Different conformations of butane are obtained by rotation.
Butane has three staggered conformers (I, III and V) .
Conformer -(III), in which the two methyl groups are as far apart as possible, is more stable than the other two staggered conformers (I and V). The most stable of the staggered conformers is called the anti conformer ( in anti conformation the angle between two methyl groups is 180º ) and the other two staggered conformers are called gauche conformers. (anti in Greek for "opposite of" gauche in French for "left"). In gauche conformation the angle between two methyl groups is 60º.
In the anti conformer, the largest substituents (CH3 and CH3) are opposite to each other; in the gauche conformer, they are adjacent. Two gauche conformers have the same energy but each is 0.9 Kcal/mole less stable than the anti conformer.
Anti and gauche conformers do not have the same energy because of the steric strain. Steric strain or steric hindrance is the strain put on a molecule when atoms or groups are large in size and due to this they are too close to each other, which causes repulsion between the electrons of atoms or groups. There is more steric strain in the gauche conformer than in the anti because the two methyl groups are closer together in the gauche conformer. Steric strain in gauche conformer is called gauche interaction.
The eclipsed conformer in which the two methyl groups are closest to each other (VI) is less stable than the other eclipsed conformers (II and IV). All these eclipsed conformers have both torsional and steric strain. Torsional strain is due to bond-bond repulsion and steric strain is due to the closeness of the eclipsing groups.
In general, steric strain in the molecule is directly proportional to the size of the eclipsing groups. Eclipsed conformer (VI) is called the fully eclipsed conformer (angle between two methyl groups is zero) whereas (II) and (IV) are called eclipsed conformers. The energy diagram for rotation about the C-2-C-3 bond of butane is shown in the Fig.
Thus the relative stabilities of the six conformers of n-butane in decreasing order is as follows:
Anti > gauche > eclipsed > fully eclipsed
(III) (I) and (II) and (VI)
Thus molecules with carbon-carbon single bonds have many interconvertible conformers. Conformers cannot be separated because they rapidly interconvert.
Although anti conformation is more stable than the gauche conformation but in some cases gauche conformation is more stable than the anti because of the intramolecular hydrogen bonding which is geometrically possible only in the gauche conformation.
In ethylene chlorohydrin also, gauche conformation is more stable than the anti conformation due to the dipole-dipole attraction between OH and Cl which is geometrically possible only in the gauche conformation.
Ex.46. Write Gauche conformation of the compound CH2Cl - CH2Cl ?
Sol. Gauche conformation of the given compound is:
(i) Mole fraction of anti and gauche form : Mole fraction of stable conformers (i.e., mole fraction of anti and gauche can be calculated if dipole moment of anti and gauche form is known.
μob = μ(anti) × xa μ(gauche) × xg
where xa = mole fraction of anti form and
xb = mole fraction of gauche form.
Suppose,
μob = 1.00
μg = 5.55
Then xa can be calculated as follows : μob = μa × xa + μg × xg
1 = 0 × xa + 5.55 xg
xg = = 0.18
Sum of mole fraction of xa + xg = 1
xa = 1 - xg = 1 - 0.18 = 0.82
(ii) relative amounts of anti and gauche conformers:
The anti conformer of n-butane is more stable than the gauche may about 900 Kcal/mole (i.e., 0.9 Kcal/mole). This is energy barrier between anti and gauche.
Thus, gauche anti, ΔH = - 900 cal/mole.
Suppose at room temp ΔG is negligible.
So ΔG = - RT ln Keq
Keq =
and ln Keq = = 1.52
The ratio of Keq is 4.57 ≈4.6, which means that about 82% mole of the molecule are in the anti conformation and 18% in the gauche conformation at any one time.
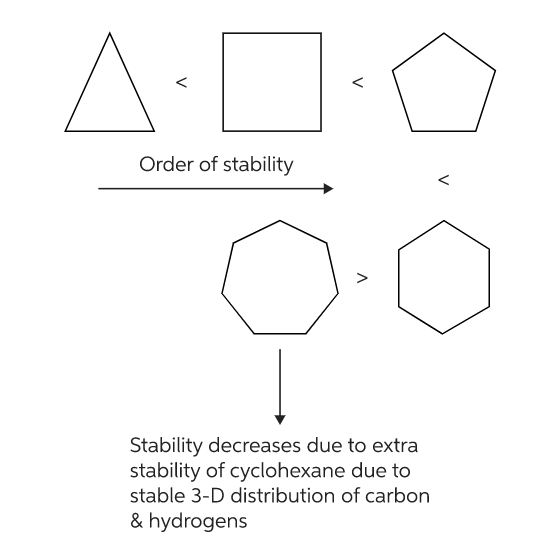
Stability of cycloalkanes:
Compounds with three and four-membered rings are not as stable as compounds with six-membered rings.
The German chemist Baeyer was the first to suggest that the instability of these small ring compounds was due to angle strain. This theory is known as the Baeyer-Strain theory.
Baeyer strain theory was based upon the assumption that when an open-chain organic compound having the normal bond angle 109.5º is converted into a cyclic compound, a definite distortion of this normal angle takes place leading to the development of a strain in the molecule.
Baeyer assumed that cyclic rings are planar. Assuming that the rings are planar, the amount of strain in various cycloalkanes can be expressed in terms of angle of deviation (d).
where n = number of carbon-carbon bonds in cycloalkane ring
a = inner bond angle in the cycloalkane ring.
Angle strain
Stability inner angle (a)
Now let us take the case of three to eight-membered cyclic compounds.
The positive and negative values of (d) indicate whether the inner angle is less than or more than the normal tetrahedral value.
Baeyer thus predicted that a five-membered ring compound would be the most stable. He predicted that six-membered ring compounds would be less stable and as cyclic compound became larger than five-membered ring they would become less and less stable.
Contrary to what Baeyer predicted, however, cyclohexane is more stable than cyclopentane. Furthermore, cyclic compounds do not become less and less stable as the number of sides increase. Thus Baeyer strain theory is applicable only to cyclopropane, cyclobutane and cyclopentane.
The mistake that Baeyer made was to assume that all cyclic compounds are planar. In real sense only cyclopropane is planar and other cycloalkanes are not planar. Cyclic compounds twist and bend in order to achieve a final structure that minimizes the three different kinds of strain that can destabilize a cyclic compound.
1. Angle strain is the strain that results when the bond angle is different from the desired tetrahedral bond angle of 109.5º.
2. Torsional strain is caused by the repulsion of the bonding electrons of one substituent with bonding electrons of a nearby substituent.
3. Steric strain is caused by atoms or groups of atoms approaching each other too closely.
Ex.47 According to Baeyer-strain theory which compound has minimum angle strain?
(A) n-butane (B) cyclopentane (C) cyclopropane (D) cyclohexane
Sol. (B)
Ex.48 Which form of trans 1,4-cyclohexane diol is most stable?
(A)
(B)
(C)
(D)
Sol. (C)
Conformation of Cyclohexane
Despite Baeyer's prediction that the five-membered cyclic compound would be the most stable, the six-membered cyclic compounds is the most stable. Six membered cyclic compounds are most stable because they can exist in a conformation that is almost completely free of strain. This conformation is called the chair conformation. In a chair conformation of cyclohexane, all bond angles are 111º which is very close to 109.5º and all the adjacent carbon-hydrogen bonds are staggered.
⇒ Each carbon in chair conformation has an axial bond and an equatorial bond.
⇒ Axial bonds are perpendicular to the plane of the ring and equatorial bonds are in the plane of the ring.
⇒ If axial bond on carbon-1 is above the plane of the ring then axial bond on carbon-2 will be below the plane of the ring. Thus, C -1 , C - 3 and C - 5 axial bonds are above and C -1 , C - 4 and C - 6 axial bonds are below.
⇒ Thus C -1 axial and C - 2 axial are trans to each other. Similarly C - 1 and C - 5 axial are cis to each other.
⇒ It axial bond on carbon - I will be above the plane then equatorial bond on this carbon will be below the plane.
(i) Thus C - 1 equatorial and C -2 equatorial will be cis
(ii) C - 1 axial and C-2 equatorial will be cis
⇒ As a result of rotation about carbon-carbon single bonds cyclohexane rapidly interconverts between two stable chair conformations. This interconversion is known as ring-flip. When the two chair forms interconvert, axial bonds become equatorial and equatorial bonds become axial.
⇒ Cyclohexane can also exist in a boat conformation. Like the chair conformation, the boat conformation is free of angle strain. However, the conformation is less stable than the chair conformation by 11 Kcal/mole. Boat conformation is less stable because some of the carbon-hydrogen bonds in boat conformation are eclipsed.
The boat conformation is further destabilised by the close proximity of the flagpole hydrogen. These hydrogen are 1.8 Å apart but the Van der Waal's radii is 2.4 Å. The flagpole hydrogen is also known as trans nuclear hydrogen.
When one hydrogen of cyclohexane is replaced by a larger atom or group, crowding occurs. The most severe crowding is among atoms held by the three axial bonds on the same side of the molecule; the resulting repulsive interaction is called 1,3-diaxial interaction.
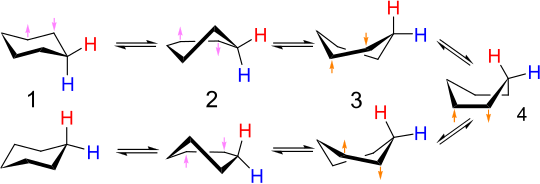
This causes steric strain in the molecule. Thus, monosubstituted cyclohexane will assume chair conformation in which the substituent occupies an equatorial position. Similarly, in di-substituted cyclohexanes, the chair conformation containing both the substituents in equatorial positions will be the preferred conformation. In general, the conformation with a bulkier substituent in an equatorial position will be the preferred conformation.
For examples:
cis 1,3-cyclohexanediol has shown to have diaxial rather than the diequatorial orientation. This is because of the stabilization orientation. This is because of the stabilization of the diaxial form by intramolecular hydrogen bonding which is not possible in the diequatoroial form.
The preferred conformation of the cyclohexane ring is the chair form, but when intramolecular hydrogen bonding is possible between groups in 1 and 4 positions the molecule assumes a boat conformation rather than the chair conformation in which this hydrogen bonding is not possible.
|
114 videos|263 docs|74 tests
|
FAQs on Conformational Isomerism - Chemistry Class 11 - NEET
| 1. What is conformational isomerism in alkanes? |  |
| 2. What is a Newman projection of ethane? |  |
| 3. What is a rotational barrier in alkanes? |  |
| 4. How does torsional strain affect the stability of alkanes? |  |
| 5. How does the conformation of cyclohexane affect its stability? |  |
















