Acids, Bases & Salts | Chemistry Class 11 - NEET PDF Download
Acids and Bases
Earlier Definitions of Acids and Bases
Earlier definitions of acids and bases was given by Robert Boyle, who classified them on the basis of their properties. According to him, acids are the substance which have sour taste. turns blue litmus red. liberate hydrogen with metals, conduct electricity in aqueous solution and neutralise bases.
Bases are the substance which have bitter taste, turns red litmus blue, soapy to touch, conduct electricity in aqueous solution and neutralise acids.
Arrhenius Concept of Acids and Bases
- Acid is a chemical substance which dissociates in aqueous solution to give hydrogen ions (H+) or hydronium ions (H3O+).
- Base is a chemical substance which dissociates in aqueous solution to give hydroxyl ions (OH–).
- Arrhenius theory fails to explain the acidic and basic behaviour in non-aqueous solutions. It cannot explain the acidic character of A1Cla. BFa and basic character of NH3, PH3, etc.
Bronsted Concept of Acids and Bases
Acid is a chemical substance that can donate a proton (H+) to some other substance and a base is a chemical substance that can accept a proton from other substance. Thus, an acid is a proton donor (protongenic) and a base is proton acceptor (protopbilic).
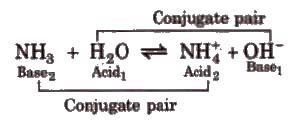
Strong acid has weak conjugate base and weak acid has strong conjugate basco Strong base has weak conjugate acid and weak base has strong conjugate acid.
HClO4 is the strongest while HCN is the weakest hydracid known.
CsOH is the strongest base known.
Amphoteric or arnphiprotic substance or ampholytes are the substance which act as an acid as well as a base, e.g.• water acts as an acid with NH3 and a base with acetic acid.
The order of acidic strength of some acids is
HClO4 > HBr> H2SO4 > HCl> HNO3
Greater the Ka value of an acid (or lesser the pKa), stronger is the acid. Similarly. greater the Kb (or lesser the pKb) of a base. stronger is the base.
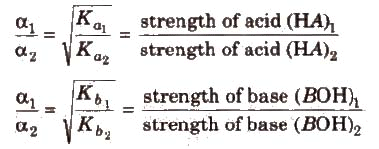
Levelling Effect
The adds like HClO4 H2SO4, HNO3 etc. react with water almost completely to form H3O+ions. Therefore, all the strong acids in aqueous solutionsappear equally strong and their relative strengths in aqueous solution cannot be compared. Since H3O+ is the strongest acid in water. the strength of above acids come down to the level of H3O+ strength in water. Similarly.strong bases like NaOH. KOH. Ba(OH)2 come down to the strength of OH– ion in water.
This is called levling effect.
Lewis Concept of Acids and Bases
Lewis acid is a chemical substance which can accept a pair of electrons,
e.g.,
- Molecules with incomplete octet of central atom like AlCl3 ,BeCl2, MgCl2, etc.
- Simple cations like Ag+, Na+, etc.
- Molecules in which the central atom has vacant d-orbital, e.g.,SF4, SnC14 PF3 etc.
Lewis base is a chemical substance which can donate a pair of electrons. e.g.,
- Neutral molecules containing lone pairs like NH3, RNH2, ROH etc.
- Negatively charged species like CN, Cl. OH, etc.
- In coordination complexes, the ligands act as Lewis base.
Limitations of Lewis Concept
- It does not explain the behaviour of protonic acids such as HCl, H4SO4, HNO3 etc.
- It does not predict the magnitude of relative strength of acids and bases.
All Bronsted-Lowry’s acids are Lewis acids while acids need not be Bronsted-Lowry’s acids.
The Ionization Constant of Water Ionic product is the product of the concentration of hydronium ions and hydroxyl ion in pure water, which remains constant at a particular temperature. It is symbolized by Kw. At 298 K, ionic product of water (KW) is given as KW: = [H3O+] [OH–] = 1 x 10-14 mol2L–.
The value of Kw increases with increase in temperature.
The pH Scale
pH is defined as the negative logarithm of hydrogen ion concentration.
pH = – log [H+] and [H+] = lO-pH
Total [H+] or [OH–] in a mixture of two strong acids or bases = (ΣNV/ΣV)
Similarly, negative logarithm of hydroxyl ion concentration is pOH.
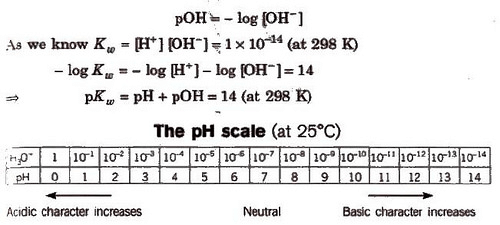
pH value of an acid having H+ concentration less than 10-7, is always in between 6 and 7. For 10-8 N HCl solution. it is 6.958.Similarly, for 10-8 NaOH solution, the pH is 7.04 (because basic solutions always have pH 77.)
pH of solution is accurately measured by pH meter or emf method or roughly by pH paper or indicator paper.
(PH can be zero in 1 N Hel solution or it can be negative for more. concentrated solution like 2N, 3N, lON, etc,
pH range for some important substances are :
Gastric juice = 1 – 3
Vinegar = 2.4 – 3.4
Tears – 7.4
Human urine – 4.8 – 8.4
Blood plasma – 7.3 – 7.4
Boil water – 6.5625
Dissociation Constant of Weak
Acid and Weak Base
Let us consider the dissociation of weak acid (HA) as
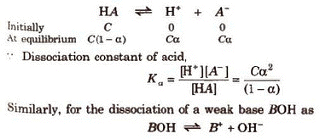
Dissociation constant for polyprotic acids and bases. For a tribasic acid,
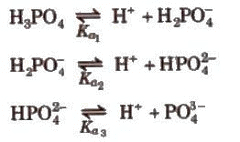
The overall dissociation constant (K) is given as
K = K1 x K2 x K3
where, K1 x K2 x K3

Similarly, for a dibasic acid like H2CO3
pH = pka1 + pka2/2)
Buffer Solution
Solution which resists the change in its pH value by addition of a small amount of acid or a base, is called buffer solution.
- Acidic buffer They have pH value < 7, e.g., CH3COOH/CH3COONa, bone acid/borax.
- Basic buffer They have pH value> 7 e.g., NH4OH/NH34Cl
Buffer system present in blood is H2CO3 + NaHCO3.
Henderson-Hesselbalch Equation
Equation used to calculate the pH of a buffer solution.
(i) For acidic buffer,
pH = pKa + log[salt/acid]
(i) pOH = pKb + log[salt/acid]
and pH = 14 – pOH
Here, pKa = -log Ka, pKb = -log Kb and Ka and Kb are dissociation constants of acid and base.
[salt), [acid] and [base) represent molar concentrations of salt, acid and base respectively.
If addition of a strong acid or base changes the pH of a buffer by/unit, the buffer solution is assumed to destroyed, i.e.,
New pH = pKo ± 1
This means the ratio.
[salt]/[acid) or [salt]/[base] = 10 or (1/10)
Buffer Capacity
It is defined as the number of moles of acid or base added in 1 L of solution Lochange the pH by unity.
Buffer capacity (φ) = (no. of moles of acids or base added to 1 L of buffer/change in pH)
Salts
These are the product of reaction between an acid and a base.
This reaction is called neutralisation reaction.
For Acidic Buffer mixture( Henderson- Hasselbalch equation)
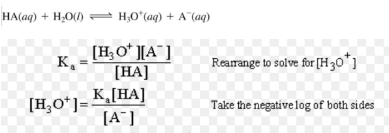
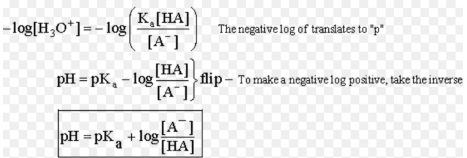
For Basic Buffer mixture ( Henderson- Hasselbalch equation)
pOH = pKb + log [salt] / [Base]
pH + pOH =14
pOH = 14 – pH
pKa + pKb = 14
pKb=14 – pKa
14 – pH = 14 – pKa + log [salt) / [base]
pH = pKa – log [salt] / [base]
pH = pKa + log [base] / [salt]
where Ka is the ionization constant of the conjugate acid of the base.
For ex: In the buffer NH4OH + NH4Cl , NH4+ is the conjugate acid of the base, NH3and Ka represents the ionization constant of the reaction:
NH4+ (aq) + H2O (l) NH3 (aq) + H+ (aq)
pH = pKa + log [NH3] / [NH4+]
pH = pKa + log [Base] / [conjugate acid]
Buffer Capacity
It is defined as the number of moles of an acid or a base required to be added to one litre of the buffer solution so as to change its pH by one unit.
Buffer Capacity = No. of moles of the acid or base added to 1 litre of the buffer / change in pH
Buffer capacity of a buffer is maximum when the concentration of the weak acid and its salt or weak base and its salt are equal i.e. when pH = pKa or pOH = pKb
Importance of Buffer Solution
1) In biological processes
The pH of our blood is maintained constant inspite of various acid and base producing reactions going on in our body.The buffer action is due to the presence of carbonic acid , bicarbonate ion and carbon dioxide in the blood.
2) In industrial processes
The use of buffers is an important part of many industrial processes, e.g.,
in electroplating, in the manufacture of leather, dyes, photographic materials.
3) In analytical chemistry
1) in the removal of acid radicals such as phosphate, oxalate and borate which interfere in the precipitation of radicals of group 3.
2) in complexometric titration
3) to calibrate the pH metres
4)In bacteriological research, culture media are generally buffered to maintain pH required for the growth of the bacteria being studied.
Types of Salts
(a) Normal salts These are obtained by complete neutralisation of an acid with a base, e.g., NaCI, K2SO4, etc.
(b) Acidic salts These are formed by incomplete neutralisation of polybasic acids. e.g., NaHCO3, Na2SO4 etc.
(c) Basic salts These are formed by incomplete neutralization of polyacidic base, e.g., Mg(OH)Cl, Bi(OH)2Cl, etc.
(d) Double salts These are formed by the combination of two simple salts and exist only in solid state, e.g., Mohr salt or ferrous ammonium sulphate (FeSO4.(NH4)2SO4.6H2O], alum, etc.
(e) Complex salts These are formed by the combination of simple salts or molecular compounds. These are stable in solid state well as in solutions.
The properties of their solutions are different from the properties of substances from which they have been constituted.
(f) Mixed salts These salts furnish more than one cation or more than one anion when dissolved in water, e.g., Ca(OCl)Cl, NaKSO4, etc.
Conjugate Acid-Base Pair(CABP) :-
In an acid-base reaction
Acid → H+ conjugate base
Base + H+ → conjugate acid.
e.g.
HCl(aq) + NH3(aq) NH4 (aq) + Cl-(aq)
Note:- A CABP is different from each other only by single proton.
e.g.
HSO-4 is the conjugate base of H2SO4 but SO2-4 is not.
Relative strength of Acids/Bases :-
Any Species and its conjugate species are opposite of each other in terms of strength. e.g.
e.g.
Strength order of acids.
HClO4 > H2SO4 > HCl > CH3COOH
strength order of conjugate bases
ClO4- < HSO4- < Cl- < CH3COO-
An ionic Equilibrium exists between the unionised electroyte molecules and the ions that result from ionisation
Types of keq
1. Self ionization of water
2. Acid dissociation constant
3. Base dissociation constant
4. Salt hydrolysis
5. Sparingly soluble salt
Factors affecting the degree of ionisation and Bronsted and Lowry Concept of Acids and Bases
Factors affecting the degree of ionization:
(a) Temperature - With the rise in temperature, the degree of dissociation of an electrolyte in solution increased. Thus, Degree of dissociation α Temperature
(b) Dilution : On the increasing of dilution, the degree of dissociation increases. But at infinite dilution, their is no effect on the degree of dissociation.
(c) Concentration of the solution :
Degree of dissociation ∝ 1/conc. of the sol. ∝ 1/amount of solute in given vol. ∝ Amount of solvent
(d) Nature of Solvent : Higher the dielectric constant of a solvent, more is its dissociation power or ionising power. Thus ,
Degree of ionisation or dissociation of an electrolyte ∝ dielectric constant of solvent.
Dielectric constant : The dielectric constant of solvent is a measure of its tendency to weaken the forces of attraction between oppositely charged ions of the given electrolyte or the force of attraction applied by solvent molecules on solute molecule is defined as Dielectric constant of solvent.
Note : Water is the most powerful ionizing solvent as its dielectric constant is highest.
(e) Presence of Common Ion : In the presence of a strong electrolyte having common ion, the degree of dissociation of an electrolyte decreases. eg. Ionisation of CH3COOH is suppressed in the presence of HCl due to common H ions.
(f) Nature of Electrolyte : At constant temperature, electrolytes ionize to a different extent in their solutions of same concentration.
Bronsted and lowry concept of acids & bases :
Postulates :-
(1) Acid - Proton (H+ ) donor
(2) Base - Proton (H+ ) acceptor
e.g.
HCl(aq) + H2O(l) H3O+ (aq) + Cl-(aq)
Acid Base ;
HCl(aq) + NH3(aq) NH4+ (aq) + Cl-(aq)
Acid Base;
HCl(aq) + CH3COOH(aq) CH3COOH+2(aq)
Acid Base Cl-(aq);
Note :- Here CH3COOH has a less tendency to donate H than HCl, therefore CH3COOH acts as a weak base.
|
119 videos|346 docs|74 tests
|






















