Bohr’s Atomic Model & Electron Orbits | Physics for JEE Main & Advanced PDF Download
Bohr’s Atomic Model
Electron can revolve in certain non-radiating orbits called stationary or bits for which the angular momentum of electron is an integer multiple of (h / 2π)
mvr = nh / 2π
where n = I, 2. 3,… called principle quantum number.
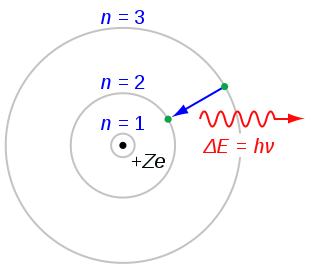 Bohr's Atomic Model
Bohr's Atomic Model
The radiation of energy occurs only when any electron jumps from one permitted orbit to another permitted orbit.
Energy of emitted photon
hv = E2 – E1
where E1 and E2are energies of electron in orbits.
Radius of orbit of electron is given by
r = n2h2 / 4π2 mK Ze2 ⇒ r ∝ n2 / Z
where, n = principle quantum number, h = Planck’s constant, m = mass of an electron, K = 1 / 4 π ε, Z = atomic number and e = electronic charge.
Velocity of electron in any orbit is given by
v = 2πKZe2 / nh ⇒ v ∝ Z / n
Frequency of electron in any orbit is given by
v = KZe2 / nhr = 4π2Z2e4mK2 / n3 h3
⇒ v prop; Z3 / n3
Kinetic energy of electron in any orbit is given by
Ek = 2π2me4Z2K2 / n2 h2 = 13.6 Z2 / n2 eV
Potential energy of electron in any orbit is given by
Ep = – 4π2me4Z2K2 / n2 h2 = 27.2 Z2 / n2 eV
⇒ Ep = ∝ Z2 / n2
Total energy of electron in any orbit is given by
E = – 2π2me4Z2K2 / n2 h2 = – 13.6 Z2 / n2 eV
⇒ Ep = ∝ Z2 / n2
Wavelength of radiation emitted in the radiation from orbit n2 to n1 is given by
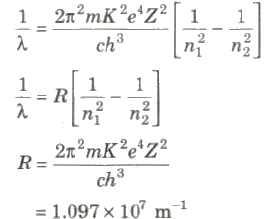
In quantum mechanics, the energies of a system are discrete or quantized. The energy of a particle of mass m is confined to a box of length L can have discrete values of energy given by the relation
En = n2 h2 / 8mL2 ; n < 1, 2, 3,…
Angular Momentum Of Electron
What is Angular Momentum of Electron?
Angular momentum of an electron by Bohr is given by mvr or nh/2π (where v is the velocity, n is the orbit in which electron is, m is mass of the electron, and r is the radius of the nth orbit).
Bohr’s atomic model laid down various postulates for the arrangement of electrons in different orbits around the nucleus. According to Bohr’s atomic model, the angular momentum of electron orbiting around the nucleus is quantized. He further added that electrons move only in those orbits where angular momentum of an electron is an integral multiple of h/2. This postulate regarding the quantisation of angular momentum of an electron was later explained by Louis de Broglie. According to him, a moving electron in its circular orbit behaves like a particle wave.
De Broglie’s Explanation to the Quantization of Angular Momentum of Electron:
The behaviour of particle waves can be viewed analogously to the waves travelling on a string. Particle waves can lead to standing waves held under resonant conditions. When a stationary string is plucked, a number of wavelengths are excited. On the other hand, we know that only those wavelengths survive which form a standing wave in the string, that is, which have nodes at the ends.
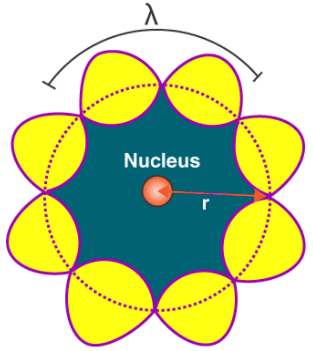 Quantization of Angular Momentum of Electron
Quantization of Angular Momentum of Electron
Thus, in a string, standing waves are formed only when the total distance travelled by a wave is an integral number of wavelengths. Hence, for any electron moving in kth circular orbit of radius rk, the total distance is equal to the circumference of the orbit, 2πrk.
2πrk = kλ
Let this be equation (1).
Where,
λ is the de Broglie wavelength.
We know that de Broglie wavelength is given by:
λ = h/p
Where,
p is electron’s momentum
h = Planck’s constant
Hence,
λ = h/mvk
Let this be equation (2).
Where mvk is the momentum of an electron revolving in the kth orbit. Inserting the value of λ from equation (2) in equation (1) we get,
2πrk = kh/mvk
mvkrk = kh/2π
Hence, de Broglie hypothesis successfully proves Bohr’s second postulate stating the quantization of angular momentum of the orbiting electron. We can also conclude that the quantized electron orbits and energy states are due to the wave nature of the electron.
|
268 videos|732 docs|171 tests
|
FAQs on Bohr’s Atomic Model & Electron Orbits - Physics for JEE Main & Advanced
| 1. What is Bohr's atomic model? |  |
| 2. What is the angular momentum of an electron in Bohr's atomic model? |  |
| 3. How does Bohr's atomic model explain electron orbits? |  |
| 4. What are the limitations of Bohr's atomic model? |  |
| 5. How does Bohr's atomic model contribute to our understanding of atomic structure? |  |

















