Air Pollution: Causes & Effects | Biology for JAMB PDF Download
| Table of contents |

|
| What is Air Pollution? |

|
| Major Air Pollutants And Their Effects: |

|
| Secondary Pollutants |

|
| Green House Effect |

|
| Ozone Depletion |

|
| Control of Air Pollution |

|
What is Air Pollution?
The air pollution is caused due to addition of unwanted substances or gases. The atmospheric pollution is mainly caused by the activities of man and concentrated to the inhabited and the industrial complexes in cities.
There are two main categories of air pollutants :
- Gases
- Particulates
 (i) Gases
(i) Gases
The gaseous materials include various gases and vapours of volatile substances or the compound with a boiling point below 200ºC.
(ii) Particulate matter
Particulate matter consist of solid particles or liquid droplets (aerosols) small enough to remain suspended in air. eg., soot, smoke, dust, asbestos, fibres, pesticides, some metals (including Hg, Pb, Cu and Fe) and also biological agent like tiny dust mites and flower pollen.
Atmospheric particles having diameter > 10 μm, generally settle down in less than a day, whereas particles with diameters 1 μm or less can remain suspended in air for weeks.
Suspended particulate matter in the lower atmosphere (troposphere) causes and aggravates human respiratory illness, like asthama, chronic bronchitis etc.
According to Central Pollution Control Board (CPCB), particulate size of 2.5 micrometers or less (diameter) (PM 2.5) are responsible for causing harm to human health as inhaled deep into lungs can cause breathing and respiratory symptoms, irritation, inflammations, damage to lungs & premature death.
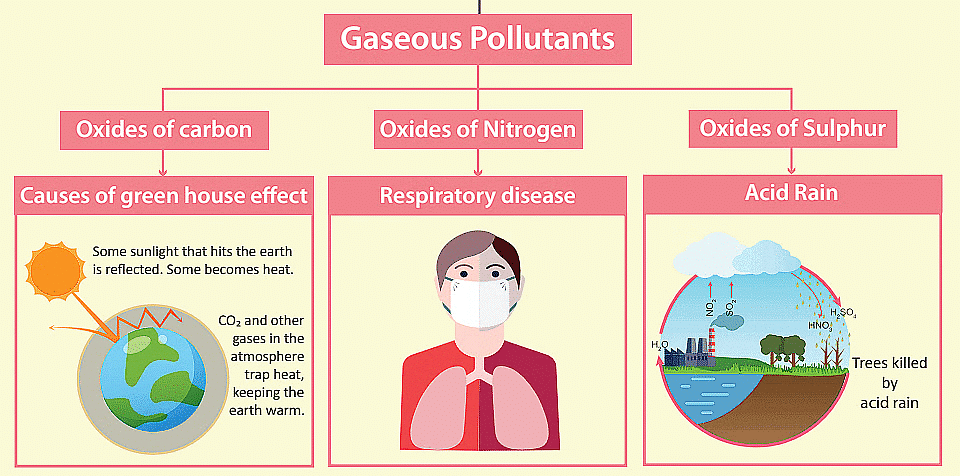
Major Air Pollutants And Their Effects:
1. Carbon monoxide (CO)
- Source - It is the main air pollutant released from smoke of automobiles.
- Effect - Carbon monoxide is a highly toxic gas, it combines with haemoglobin of the blood and blocks the transportation of oxygen. Thus, it impairs respiration and it causes death due to asphyxia when inhaled in large amount.
2. Unburn Hydrocarbons - (3,4 Benzopyrine, CH4, Benzene)
- Source - These are mainly released from automobiles and burning of fossil fuel (coal, petrol, diesel). Methane (CH4) is the most abundant hydrocarbon in atmosphere and its main source is marshy area and paddy fields.
- Effect - Hydrocarbons cause lungs cancer.
- Polynuclear hydrocarbon is major hydrocarbon pollutant which causes cancer.
3. Ethylene
- Source - Its main sources are automobiles, chimneys.
- Effect - Falling of leaves without particular reason, falling of flowering bud before time.
4. Nitrogen oxide (NO, NO2)
- Source - Burning (combustion) of fossil fuel in automobiles.
- Effect - These nitrogen oxide form photochemical smog in atmosphere and release ozone.
Nitrogen oxide is also responsible for acid rain. Entry of nitrogen oxide causes respiratory trouble such as emphysema, bronchitis, swelling of lungs and lungs cancer etc.
5. Sulphur oxide (SO2, SO3)
- Source - These are most harmful gaseous pollutants. Main source of sulphur oxides are coal burning, smelters, oil refineries.
- Effect - Lichen and mosses do not grow in SO2 polluted areas, Lichen and mosses are indicator of SO2 pollution. Oxides of sulphur produce acid rain and smog in atmosphere.
6. Smoke - (SO2, SO3, NO2, NO, CO, CO2)
Oxides of Sulphur
- General Term: SOx refers to all sulphur oxides with major ones being sulphur dioxide (SO2) and sulphur trioxide.
- Sulphur Dioxide: SO2 is a colorless, pungent gas that dissolves in water to form sulphurous acid.
- Formation: SO2 reacts with oxygen in air slowly to form sulphur trioxide (SO3), which further combines with water to form sulphuric acid.
- Reactions:
- 2SO2 (g) + O2 (g) → 2SO3 (g)
- SO2 (g) + O3 (g) → SO3 (g) + O2 (g)
- SO2 (g) + H2O2 (l) → H2SO4 (aq)
- Source: Burning of sulphur-containing fuels leads to their formation, harmful to living organisms.
Effects:
- On Humans: SO2 can cause respiratory issues, eye irritation, and even bronchitis and lung cancer.
- On Plants: Interferes with chlorophyll synthesis, reduces plant yield, and harms vegetation.
Oxides of Nitrogen
- Types: Nitric oxide (NO), nitrogen dioxide (NO2), and nitrous oxide (N2O) are key nitrogen oxides.
- Formation: Result from natural and human activities, forming acid rain and photochemical smog.
- Reactions:
- N2 (g) + O2 (g) → 2NO(g) at 1483 K
- 2NO (g) + O2 (g) → 2NO2 (g)
- NO (g) + O3 (g) → NO2 (g) + O2 (g)
Effects:
- On Health: Causes respiratory diseases, eye irritation, and lung infections in humans, especially affecting children and the elderly.
- On Vegetation: Damages plant leaves, suppresses growth, and leads to chlorosis.
- Environmental Impact: Causes acid rain, damages materials, and contributes to photochemical smog formation.
Secondary Pollutants
(i) Smog (Smoke Fog)
Definition: The term "smog," a combination of smoke and fog, was coined by Desvoeux. It is quantified using the Ringlmann method.
Types of Smog:
- Los Angeles Smog or Photochemical Smog: Initially observed in Los Angeles, this smog variation involves smoke, fog, nitrogen oxides, hydrocarbons, oxygen, UV light, and high temperatures. These elements interact to produce reddish-brown smog or brown haze, including components like PAN, O3, and nitrogen oxides. Known as light-induced smog.
- London Smog or Sulphur Smog: First identified in London, this type results from the interplay of coal, smoke, fog, sulphur oxides, and low temperatures, creating a vapor of H2SO4, termed London smog.
Effects of Smog:
- Smog detrimentally impacts elastic materials like rubber and tires. Peroxyacetyl nitrate (PAN) is generated during smog, inhibiting water photolysis in photosynthesis and chlorophyll formation, along with causing eye irritation and lung damage in animals.
- Ozone, a component of smog, harms mucous membranes.
- In London, the inhalation of H2SO4 vapor during the 1952 smog incident led to the deaths of approximately 4000 individuals.
(ii) Acid rain
This term was coined by Robert August. It occurs when NO2 and SO2 are emitted from various sources in the form of smoke and then dissolve in atmospheric water vapor, leading to the formation of sulphuric acid and nitric acid (H2SO4, HNO3).
When these acids combine with rainwater and fall to the earth's surface, it is termed as acid rain.
Types of deposition
- Wet deposition: Occurs when acid falls to the earth through rain, fog, and smog.
- Dry deposition: Happens when acid settles on the earth's surface through solid dust particles containing nitrate or sulphate.
Note
- The pH level of acid rain ranges from 3.5 to 4, whereas normal rain has a pH of 5.6.
- In acid rain, the ratio of H2SO4 to HNO3 is 7:3 (70% H2SO4, 30% HNO3).
Effects of Acid Rain
- Acid rain leads to an increase in the acidity of soil and water.
- It also causes damage to historical monuments such as the Taj Mahal and the Red Fort.
Green House Effect
The greenhouse effect is the process through which heat is trapped near Earth's surface by substances known as 'greenhouse gases.' Imagine these gases as a cozy blanket enveloping our planet, helping to maintain a warmer temperature than it would have otherwise. Greenhouse gases consist of carbon dioxide, methane, ozone, nitrous oxide, chlorofluorocarbons, and water vapor.
Effect of Greenhouse Gases on Earth's Temperature
- Before Greenhouse Effect: Without the greenhouse effect, the average temperature of the Earth's surface would have been -18°C instead of the current 15°C.
- Greenhouse Effect Explanation: Normally, carbon dioxide is not classified as a pollutant. However, when its concentration increases, it forms a dense layer in the atmosphere that traps heat radiation from the Earth's surface. As a result, the temperature of the Earth's surface rises, leading to what is known as the "Greenhouse Effect" or Global Warming.
- Contribution of Greenhouse Gases: The primary greenhouse gases contributing to global warming are carbon dioxide (CO2), methane (CH4), chlorofluorocarbons (CFC), and nitrous oxide (N2O). These gases are capable of absorbing long-wave infrared radiation. On the other hand, sulfur dioxide (SO2), nitrogen dioxide (NO2), ozone (O3) do not contribute significantly to the greenhouse effect. Water vapors released from industrial and agricultural activities also play a role in intensifying the greenhouse effect.
Global Warming
In this phenomenon, cover of CO2 layer around the earth, allow the short wavelength (U.V. rays) incoming solar radiation to come in but does not allow the long wavelength (IR) of out going heat radiation from warm surface of earth to keep earth warm. The consequent increase in the global mean temperature is referred to as global warming.
Effects of Greenhouse Gases
- It has been observed that the level of CO2 in the atmosphere has increased significantly over the years. The amount of CO2 is projected to double by 2020 if the current growth rate continues.
- A temperature rise of 2-3°C could result in the melting of glaciers and ice caps, leading to floods, sea level rise, and changes in rainfall patterns. Some islands may even be submerged in seawater. The concentration of CO2 in 2009-2010 was measured at 385 ppm.
- The Carbon Dioxide fertilization effect occurs as a result of increased CO2 levels, leading to enhanced photosynthesis rates in plants for a few years.
- This phenomenon benefits C3 plants under optimal environmental conditions, reducing stomatal conductance and potentially lowering transpiration rates, thereby increasing water use efficiency. The higher photosynthesis rate under elevated CO2 levels can boost root growth, mycorrhizal development, and nitrogen fixation in nutrient-poor soils.
- The global mean temperature witnessed a 0.6°C increase in the 20th century, alongside concerns about ozone layer depletion in the stratosphere. Moreover, sea levels rose by 1 to 2 mm annually during the same period.
Control of Global Warming
- UNCED (United Nations Conference on Environment and Development) Earth Summit took place in Rio de Janeiro (Brazil) in 1992 to address the reduction of greenhouse gases and biodiversity conservation and to establish Agenda 21.
- The Kyoto Protocol conference was held in Kyoto (Japan) in 1997 to combat climate change. This protocol mandated countries to implement measures aimed at reducing their total greenhouse gas emissions to a level 5% below the 1990 benchmark by the commitment period of 2008-2012.
Earth Summit or world summit on sustainable development (2002) was held in Johanncsburg (S.Africa).
1. Reducing the green house gases emission by limiting uses of fossil fuels and developing alternative renewable sources of energy (wind and solar energy).
2. Increasing the vegetative cover mainly forests for photosynthetic utilization of CO2.
3. Minimizing the use of Nitrogen fertilizers in agriculture for reducing N2O emission.
4. Developing substitute for CFC's.
Ozone Depletion
Ozone is found in lower quantities in the atmosphere, but its concentration peaks at 16 to 25 km in the stratosphere. Naturally, there are two types of ozone: 'bad' ozone, produced in the lower atmosphere (troposphere), which is harmful to plants and animals, and 'good' ozone, located in the upper stratosphere, acting as a shield against ultraviolet radiation from the sun.
- Ultraviolet (UV) rays pose a significant threat to living organisms as they can damage DNA and proteins by breaking chemical bonds due to their high energy. The thickness of ozone in the atmosphere is measured in Dobson units (DU).
- Ozone is continually generated in the stratosphere through the interaction of UV rays with molecular oxygen. Maintaining a balance between ozone production and degradation is crucial. However, this balance has been disrupted recently by the increased degradation of ozone caused by substances like chlorofluorocarbons (CFCs), methane (CH₄), and nitrous oxide (N₂O).
- CFCs are commonly used as refrigerants. When CFCs are released into the lower atmosphere, they rise and reach the stratosphere. There, UV rays break them down, releasing chlorine (Cl) atoms. These Cl atoms then contribute to ozone depletion by releasing molecular oxygen, acting as catalysts in the process without being consumed themselves.
In this process one chlorine atom convert each O3 molecule into O2 by photo dissociation.
The life time of CF2Cl2 (CFC-12) is 139 years while that for CFCl3 (CFC = 11) is about 77 years.
Hence, whatever CFCs are added to the stratosphere, they have permanent and continuing effects on Ozone levels. although ozone depletion is happening widely in the stratosphere, it is particularly severe over the Antarctic region, leading to the creation of a large area of thinned ozone layer known as the ozone hole.
- U.V. radiation of wavelengths shorter than UV-B is largely absorbed by Earth's atmosphere when the ozone layer is intact. However, UV-B can harm DNA, leading to mutations. It also causes skin aging, damage to skin cells, and various types of skin cancers like Melanoma. In the human eye, the cornea absorbs UV-B radiation, and excessive exposure can result in inflammation of the cornea, known as snow blindness, cataracts, and other issues. Prolonged exposure may cause permanent damage to the cornea.
- Recognizing the harmful impacts of ozone depletion, an international treaty called the Montreal Protocol was signed in Montreal, Canada, in 1987 (effective in 1989) to regulate the emission of ozone-depleting substances.
Control of Air Pollution
Two devices are used to remove particulate air pollutants:
- Arresters
- Scrubbers
Arresters
These devices are employed to separate particulate matters from contaminated air.
(a) Cyclonic separators and Trajectory separators: These are commonly utilized for separating out particulate matters from industrial emissions with minimal moisture content. They operate based on the principle of dust separation through centrifugal force.
(b) Electrostatic Precipitator:
An electrostatic precipitator (ESP) is a device that utilizes electrical charges to remove certain impurities, such as solid particles or liquid droplets, from air or other gases in industrial settings. It is commonly used in smokestacks and flues to clean exhaust gases. The ESP operates by applying energy directly to the particulate matter, which allows for efficient collection without significantly restricting the flow of gases.
Function and Operation
- The primary function of an electrostatic precipitator is to eliminate unwanted particulate matter from industrial emissions. This process involves charging the particles and then using an electric field to collect them on plates or other collection surfaces.
- The collected particles are then removed from the system. Originally developed to recover valuable materials from industrial processes, ESPs are now widely used for air pollution control.
Applications and Benefits
- Electrostatic precipitators are vital in reducing harmful particulate emissions from industrial facilities and power plants. By capturing fine particles, particularly those smaller than 2.5 microns in diameter, ESPs play a crucial role in preventing air pollution.
- If these fine particles are released into the atmosphere, they can decrease visibility, contribute to climate change, and pose serious health risks, including lung damage and bronchitis, as they can penetrate deep into the respiratory system and cause inflammatory reactions.
Importance in Pollution Control
The ability of electrostatic precipitators to capture tiny, potentially hazardous particles makes them an essential component in efforts to control air pollution and protect public health. By preventing the release of fine particulate matter into the environment, ESPs help to maintain cleaner air, thereby reducing the incidence of respiratory illnesses and minimizing the environmental impact of industrial activities.
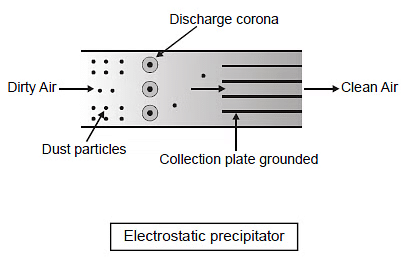
Key Features of Electrostatic Precipitator:
- Can eliminate 99% of particulate matter found in exhaust from a Thermal Power Plant.
- Consists of electrode wires (acting as anodes) maintained at several thousand volts, inducing corona and releasing electrons.
- These electrons attach to dust particles, giving them a negative charge.
- The base contains collecting plates (acting as cathodes) that attract the charged dust particles.
- Low-velocity air is provided between the plates, allowing the dust to settle.
Scrubbers
Scrubbers are another type of device used for particulate matter control. These are used to clean air for both dust and gases. Wet and dry two types of scrubbers are used for dust separation. They can remove gases like sulphur. In scrubber the exhaust is passed through a spray of water or lime.
Control Techniques for Gaseous Pollutants
- Combustion: In this method, oxidizable gaseous pollutants are completely burned at high temperatures. Industries like petrochemical, fertilizer, paints, and varnish utilize combustion to control gaseous pollutants.
- Absorption: Gaseous pollutants are absorbed in suitable absorbent materials in this technique.
- Adsorption: This method is used to control toxic gases, vapors, and inflammable compounds that cannot be efficiently removed by other techniques. These air pollutants are adsorbed on large solid surfaces.
- Catalytic Converters: Automobiles are a significant source of atmospheric pollution in metropolitan areas. Catalytic converters, containing expensive metals like platinum, palladium, and rhodium, are installed in vehicles to reduce emission of harmful gases. Unburnt hydrocarbons are converted into CO2 and water, while CO and nitric oxide are transformed into CO2 and nitrogen gas as they pass through the catalytic converter. Vehicles with catalytic converters should use unleaded petrol to prevent catalyst deactivation by lead in petrol.
These techniques play a crucial role in reducing gaseous pollutants and improving air quality.
Some other Method
1. Engines should not be kept started when vehicle is in rest condition.
2. Barium compound mixed with petrol reduce the smoke.
3. It is also very essential to check the quality of gases released from the factories.
4. Industries should not be established at one place.
5. The smoke should be released into the atmosphere after filtration and purification (by cyclone collector or electrostatic precipitators).
6. To separate particles larger than 50 μm, gravity settling tanks or porous filters are being used.
Controlling Vechicular Air Pollution : (A case study of Delhi)
- Delhi's Air Pollution: Delhi, with a high volume of vehicles, is a major hub of air pollution in India.
- Past Pollution Levels: In the 1990s, Delhi ranked fourth among the most polluted cities globally.
- Government Intervention: A Public Interest Litigation led to the conversion of public transport to CNG by 2002.
- CNG Advantages: CNG is preferred for its efficient combustion and lower chances of theft or adulteration.
- Challenges with CNG: Challenges include setting up pipelines for distribution and ensuring a steady supply.
- Steps for Pollution Control: Delhi implemented measures like phasing out old vehicles and using cleaner fuels.
- Auto Fuel Policy: India's policy aims to reduce pollution by setting stringent norms for fuel quality.
- Future Regulations: Bharat Stage-II norms will be enforced nationwide from April 2005.
|
225 videos|175 docs|156 tests
|
FAQs on Air Pollution: Causes & Effects - Biology for JAMB
| 1. What is the main cause of air pollution? |  |
| 2. What are the major air pollutants and their effects? |  |
| 3. How does the greenhouse effect contribute to air pollution? |  |
| 4. What is ozone depletion and how does it affect air quality? |  |
| 5. How can air pollution be controlled? |  |















