Cleansing Agents: Soaps & Detergents | Chemistry for JEE Main & Advanced PDF Download
| Table of contents |

|
| Cleansing Agents |

|
| Soaps |

|
| Detergents |

|
| Chemistry in Colouring Matter |

|
| Chemistry in Cosmetics |

|
Cleansing Agents
The word detergent means cleansing agent. Actually detergent word is derived from Latin word ‘detergere’ means “to wipe off”, Cleansing agents are the substance which remove dirt and have cleansing action in water. These are also called surfactants.
Detergents can be classified into two types.
- Soapy detergents or soaps, and
- Non-soapy detergents or soapless soap.
Soaps
Soaps are sodium or potassium salts of higher fatty acids (containing 15-18 carbon atoms) e.g., stearic acid, oleic acid and palmitic acid. Sodium salts of fatty acids are known as hard soaps while the potassium salts of fatty acids are known as soft soaps.
Hard soaps are prepared by cheaper oil and NaOH while soft soaps are Jlrepared by oil of good quality and KOH. The soft soaps do not contain freealkali, produce more lather and are used as toilet soaps, shaving soaps and shampoos.
Preparation of soaps
Soaps containing ‘Sodium salts are formed by heating fat (glyceryl ester ~fatty acid) with an aqueous sodium hydroxide solution. This reaction is known as saponification.
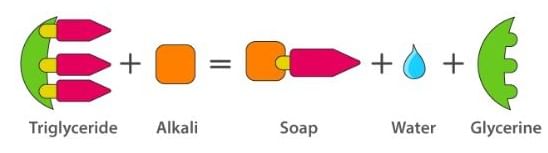
The fatty acids are later purified by the method of distillation and neutralized with an alkali to produce water and soap.
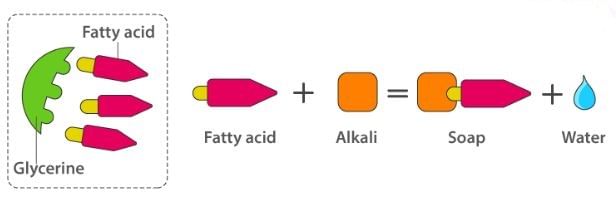
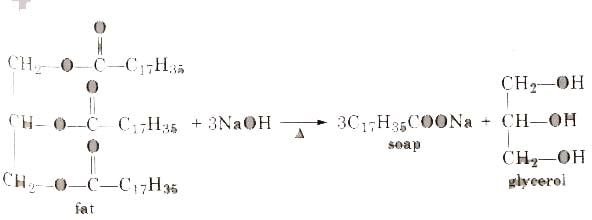
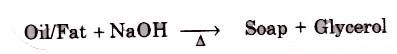
The solution left after removing the soap contains glycerol, which can be recovered by fractional distillation. To improve the quality of soaps desired colours, perfumes and medicinal chemical substances, added.
Types of Soaps
Different kind of soaps are made by using different raw materials.
- Toilet soaps These are prepared by using better grade of fat or oil and care is taken to remove excess alkali. Colour and perfumes are added to make these more attractive.
- Floating soaps These can be prepared by beating tiny bubbles into the product before it hardens.
- Transparent soaps These are made by dissolving the in ethanol and then evaporating the excess solvent.
- Medicated soaps Medicated soaps are prepared by some antiseptics like dettol or bithional.
- Shaving soaps These contain glycerol to prevent drying. A gum called rosin is added while making them. It forms sodium rosinate which lather well.
- Laundry soaps These sodium silicate, borax and contain sodium fillers like carbonate. sodium rosins
- Soap Chips These are made by running a thin sheet of melted soap on a cool cylinder and scraping off the soaps in small broken pieces.
- Soap grannules These are dried miniature soap bubbles.
- Soap powder and scouring soaps These contain a scouring agent (abrasive) such as powdered pumice or finely divided sand and builders like sodium carbonate and trisodium phosphate. Builders make the soaps act more quickly.
Disadvantages of Soaps
Soap is good cleansing agent and is 100% biodegradable microorganisms present in sewage water can completely oxidise soap to CO2, As a result, it does not create any pollution problem. However soaps have two disadvantages:
(i) Soaps cannot be used in hard water since calcium magnesium ions present in hard water produce curdy precipitates of calcium and magnesium soaps.
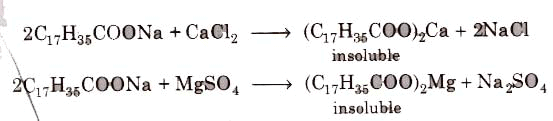
These insoluble soaps separate as scum in water and causes hinderance to washing because the precipitate adheres onto the fibre of the cloth as gummy mass. Thus, a lot of soap is wasted if water. is hard.
(ii) Soaps cannot be used in acidic solutions since acids precipitate the insoluble free fatty acids which adhere to the fabrics and thus, reduce the ability of soaps to remove oil and grease from fabrics.

Detergents
Synthetic detergents have all the properties of soaps but actually does not contain any soap, so they are known as ‘soapless soaps’.
Straight chain alkyl group containing detergents are biodegradable whereas branched chain alkyl group containing detergents are non-biodegradable.
Unlike soaps, synthetic detergents can be used in both soft and hard water. This is due to the reason that calcium and magnesium salts of detergents like their sodium salts are also soluble in water. Synthetic detergents are mainly classified into three categories:
Anionic Detergents
These are sodium salts of sulphonated long chain alcohols or hydrocarbons.
(i) Alkyl hydrogen sulphates formed by treating long chain alcohols with concentrated sulphuric acids are neutralised with alkali to form anionic detergents.
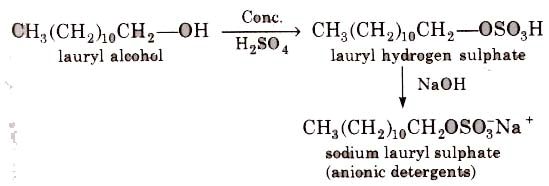
(ii) Alkyl benzene sulphonates are obtained by neutralising alkyl benzene sulphonic acids with alkali.
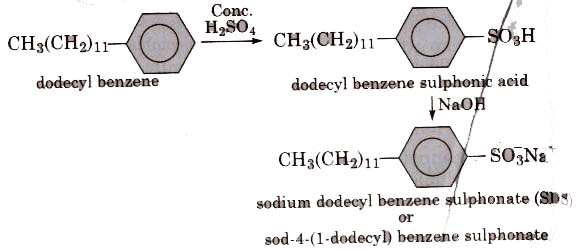
In such detergents, the anionic part of the molecule is involved in the cleansing action.
They are mostly used for household work and in toothpaste
Cationic Detergents
These are quaternary ammonium salts of arnines with acetates, chlorides or bromides as an anion. For example,
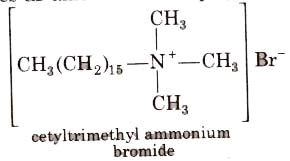
Cationic detergents are used in hair conditioner. They have germicidal properties but are expensive therefore, these are of limited use.
Non-ionic Detergents
Such detergents does not contain any ion in their constitution. One such detergent can be obtained by reaction of stearic acid and polyethylene glycol.

Liquid dish washing detergents are non-ionic type, Mechanism of cleansing action of this type of detergents is the same as that of soaps.
Advantages of synthetic detergents over soaps
1. Synthetic detergents can be used even in case of hard water whereas soaps fail to do so.
2. Synthetic detergents can be used in the acidic medium while soaps cannot because of their hydrolysis to free acids.
3. Synthetic detergents are more soluble in water and hence, form better lather than soaps.
4. Synthetic detergents have a stronger cleansing action than soaps.
Chemistry in Colouring Matter
The natural or synthetic colouring matter which are used in solution to stain materials especially fabrics are called dyes.
All colouring substances are not dyes, e.g., azobenzene, a coloured substance does ‘not act as dye.
A dye have following characteristics :.
- It must have a suitable colour.
- It can be fixed on the fabric either directly or with the help of mordant.
- It must be resistant to the action of water, acid and alkalies. The groups, responsible for colour, are called chromophore, e.g.,
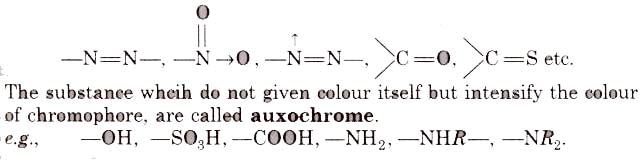
Classification of Dyes on the Basis of Constitution
(i) Nitro or nitroso dye Chromophore NO2 or NO group, Auxochrome = -OH group, e.g., picric acid, martius yellow, Gambine, naphthol yellow-S.
(ii) Azo dye, e.g., bismark brown, methyl orange, methyl red, congo red, etc.
(iii) Anthraquinone dye e.g., alizarin
(iv) Indigo is the oldest known dye. Other examples are tyrian purple, indigosol.
(v) Phthalein dye e.g., phenolphthalein, fluorescein, eosin, mercurochrome.
(vi) Triarybnethane dye, e.g., malachite green, rosaniline.
Classification of Dyes on the Basis of Application
(i) Direct dyes These dyes applied directly to fibre and are more useful to the fabrics containing H-bonding like cotton, rayon, wool, silk and nylon, e.g., martius yellow, congo reu/etc.
(ii) Acid dyes These are water soluble and contain porar/acidic groups which interact with the basic group of e.g., Orange-I, congo red, methyl orange, etc. These dyes does not have affinity for cotton but are used for silk, wool, etc.
(iii) Basic dyes These dyes contain basic group (like NHz group) and react with anionic sites present on the fabric. These are used to dye nylons and polyester, e.g., butter yellow, magenta (rosaniline), aniline yellow, etc.
(iv) Vat dyes Being water insoluble, these cannot be applied directly. These are first reduced to a colourless soluble form by a reducing agent in large vats and then, applied to fabrics. After applying, these are oxidised to insoluble coloured form by exposure. to air or some oxidising agents, e.g., Indigo, tyrian purple, etc.
(v) Mordant dyes These are applied with the help of a binding material (e.g., metal ion, tannic acid or metal hydroxide) called mordant. Depending upon the metal ion used, the same dye can give different colours. Alizarin is an important example of such dyes.
(vi) Ingrain dye These dyes are synthesised directly on the fabric. These are water insoluble and particularly suitable for cotton fibres. Azo dyes belong to this group of dye.
Chemistry in Cosmetics
Cosmetics are used for decorating, beautifying or improving complexion of skin. Some of the cosmetics of daily use are as
1. Creams
These are stable emulsions of oils or fats in water and contain emmollients (to prevent water loss) and humectants (to attract water) as two fundamental components.
2. Perfumes
These solutions have pleasent odour and invariably consist of three ingredients: a vehicle (ethanol + H20), fixative e.g., sandalwood oil, benzoin, glyceryl diacetate etc.) and odour producing substance (e.g., terpenoids like linalool, anisaldehyde (p-methoxy- benzaldehyde etc.)
3. Talcum Powder
It is used to reduce irritation of skin. Talc (Mg3(OH)2Si4O10), chalk, Zno, zinc sterate and a suitable perfume are the constituents of talcum powder.
4. Deodorants
These are applied to mask the body odour. These possess antibacterial properties. Aluminium salts, ZnO, Zn02, (C17H35COO)2Zn can be used in deodorant preparation.
Rocket Propellants
Substances used for launching rockets are called rocket propellants. These are the combination of an oxidiser and a fuel.
Depending upon the physical states of oxidiser and fuels, rocket propellants are classified as
1. Solid Propellants
These are further divided into two classes
(i) Composite propellants In these propellants, fuel is polymeric binder such as polyurethane or polybutadiene and oxidiser is ammonium per chlorate or potassium perchlorate.
|
334 videos|660 docs|300 tests
|
FAQs on Cleansing Agents: Soaps & Detergents - Chemistry for JEE Main & Advanced
| 1. What are soaps and detergents and how do they differ? |  |
| 2. How do soaps and detergents work as cleansing agents? |  |
| 3. Can soaps and detergents be used interchangeably? |  |
| 4. Are there any environmental concerns associated with soaps and detergents? |  |
| 5. Can soaps and detergents be used in cosmetics? |  |
















