Valence Bond Theory - Chemical Bonding | Inorganic Chemistry PDF Download
| Table of contents |

|
| Valence Bond Theory |

|
| Hybridization Of Atomic Orbitals |

|
| Orbital Analysis of Hybridization |

|
| Some Solved Questions |

|
Valence Bond Theory
According to this theory, a covalent bond is formed by the overlapping of two atomic orbitals of proper energy and proper symmetry. This theory was given by Hietler and London and extended by Pauling and Slater.
Hietler and London Concept of Covalent Bonding:
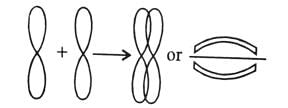
For a covalent bond to form, two atoms must come closer to each other so that orbital of one atom overlaps with the other.
- Overlapping orbitals must have:
(a) Half-filled nature, i.e., must have unpaired electron.
(b) antispin electrons.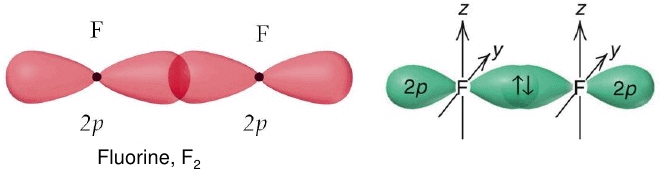 Valence bond theory
Valence bond theory - As a result of overlapping, a new localized bond orbital is formed, in which the probability of finding electron pair is maximum.
- Covalent bond energy arises due to
(a) Electrostatic attraction between nuclei and the accumulated electron cloud.
(b) Cancellation or attraction between spins of antispin electrons.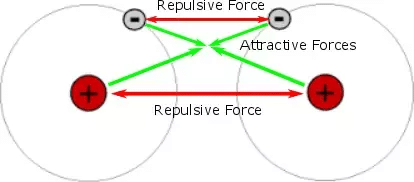
- Greater is the overlapping, lesser will be the bond length, more will be the attraction and more will be the 7 bond energy and the stability of the bond.
Pauling and Slater Extension of Covalent Bonding:
The extent of overlapping depends upon:
(a) nature of orbitals involved in overlapping.
(b) nature of overlapping.
- More closer the valence shells are to the nucleus, more will be overlapping and the bond energy will also be high. Bond energy of 1 – 1 > 1 – 2 > 2 – 2 > 2 – 3 shells.
- Between two sub-shells of the same energy level, the sub-shells more directionally concentrated shows more overlapping.
Bond energy: 2s-2s < 2s-2p < 2p-2p. - s-orbitals are spherically symmetrical and thus show only head-on overlapping. On the other hand, p-orbitals are directionally concentrated and thus, show either head-on overlapping or lateral overlapping.
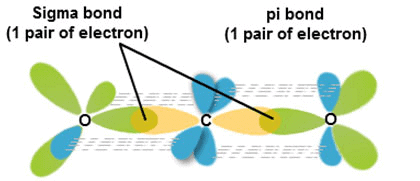
Formation of σ-bond:
(σ-Bond is formed by the axial overlapping of atomic orbitals)
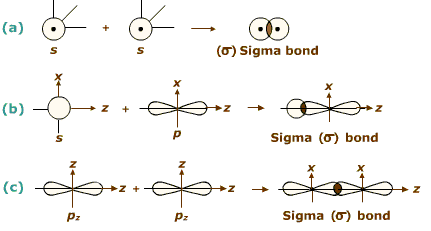 Formation of sigma bond
Formation of sigma bond
Formation of π-bond:
(p-bond is formed by the sideways overlapping of atomic orbitals)
- Head-on overlapping is stronger than lateral or sideways overlapping.
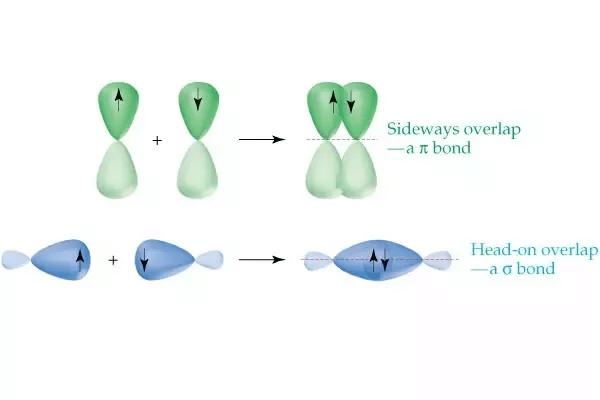
Following overlappings are not allowed
(a) (i) Intermolecular distance (x) repulsion
P.E.
stability
(ii) Also in an s-orbital, ψ is positive throughout but in p-orbital it is positive and negative
∴ Total overlapping will zero
not allowed.
Limitations of Valence Bond Theory: This theory could not explain the following facts:
(i) It fails to explain the paramagnetic behaviour of O2 molecules.
(ii) It fails to explain the bonding in electron deficient compounds as well as in metals and intermetallic compounds.
(iii) It lacks in mathematical explanation of most chemical species, as the valence bond concept is very close to the classical chemical picture. In classical chemical picture, a bond is denoted by a line whereas in valence bond theory, the electron pair plays the same role.
Hybridization Of Atomic Orbitals
The concept of hybridization lies in its origin in VBT.
Requirement of the concept of hybridization:
In VBT, the ideal of hybridization was required to explain the faces:
(i) the number of bonds formed,
(ii) the equivalence of the bonds in some cases,
(iii) the stereochemistry of the molecules,
(iv) the better overlapping of the orbitals. 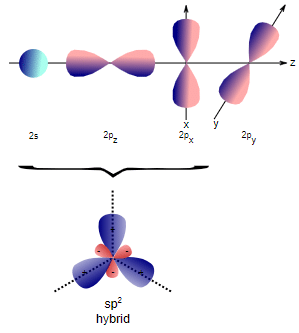 Formation of sp2 hybridization
Formation of sp2 hybridization
- To clarify the fact, let us take the most classical example, methane (CH4). Experimentally the four equivalent C–H bonds are found to project the four corners of a regular tetrahedron.
- The central atom, carbon is known to have the electronic configuration in the ground state as 1s2 2s2 2p2. It indicates that there are only two unpaired electrons in the valence shell to form two covalent (electron pair) bonds in the light of VBT. To form four bonds, it should have four unpaired electrons which can only be attained through excitation.
- In the excited state, the four unpaired electrons reside in four orbitals which have different spatial orientations.
- If these pure orbitals or carbon undergo overlapping with the 1s orbitals of hydrogens separately, then three C–H bonds formed by the three p orbitals of carbon should be mutually perpendicular.
- The fourth C–H bond formed by the 2s orbitals of carbon should lie nondirectionally as the orbital is spherically symmetrical. This is why, to explain the four equivalent bonds having bond angles 109.5º, the four orbitals, i.e. 2s2 2px1 2py1 2pz1, are to be blended or mixed up to form four equivalent orbitals having 2s property 25% in each.
- These orbitals are called sp3 hybrid orbitals and these are projected at the four vertices of a regular tetrahedron.
- In the whole process, it requires excitation energy (~406 kJ mol–1) to produce four unpaired electrons from the ground state.
- This excitation energy must be compensated by the release of energy due to the formation of four C–H bonds by using the hybrid orbitals.
Calculation of Hybridisation:
- Steric no. = 1/2 [V + M ± Q]
- V = volume shell electron of the central atom
- M = No. of monovalent atoms (H, X)
- Q = Charge on species (+ for anionic species; – for cationic species)
Rules for hybridisation
- Orbitals of similar energies belonging to the same atom or ion can hybridize together.
- The number of hybrid orbitals produced are equal to the number of orbitals undergoing hybridization. Hybrid bonds are stronger than the single non-hybridized bonds of comparable energy.
- Most of the hybrid orbitals are similar but they are not necessarily identical in shape. They differ from one another in their orientation in space.
- For equivalent hybrids, the orientation in space is determined by
(a) the number of orbitals mixed and consequently, the number of hybrids obtained, and
(b) Which of x-, y- and z-axes are preferred by the orbitals when pure. - From the type of hybridization, one can predict the geometry and bond angles of a molecule.
- An orbital that has been used to build up a hybrid orbital is no longer available to hold electrons in their pure form.
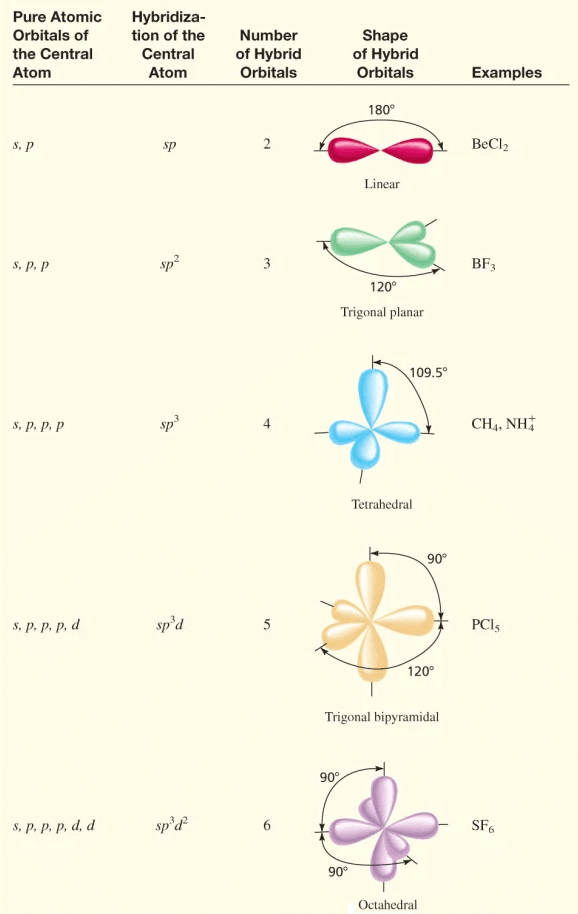 Table of hybridisation
Table of hybridisation
Equivalent hybrid orbitals
If all the hybrid orbitals after hybridization are equivalent to one another in all the manners then it is called equivalent hybrid orbitals.
For example: in the case of sp3, s + px + py + pz produce four equivalent orbitals.
Non-equivalent hybrid orbitals
In sp3d, the five hybrid orbitals are not equivalent. The three hybrid orbitals arising from s, px, and py are equivalent lying at an angle 120º in the xy-plane. The other two hybrid orbitals made of pz and d z2 are projected mutually at 180º, and perpendicular to the xy-plane.
The sp3d3 hybridization (pentagonal bipyramid) can also be analyzed in the same way giving rise to two different sets of hybrid orbitals: leading to a pentagon in the xy-plane; (pz +
) leading to a linear segment perpendicular to the pentagon.
Drago’s Rule
According to the rule whenever P and onwards elements or attach to H atom, in such compounds lone pair generally do not participate in hybridization.
Example: PH3; H2Se, AsH3, SbH3, BiH3, H2S, H2Te, etc.
Applications of Hybridization:
(i) Determination of Lewis basic strength: Hybrid orbitals shows better basic character than unhybridized orbitals as they have more directional character.
Example:
NH3 is a better Lewis base than PH3 because the lone pair in NH3 is present in sp3 hybridized state while in PH3 s orbital is unhybridized.
Similarly:
(a) NH4+ forms easily but not PH4+
(b) NH3 readily reacts with H2O but not PH3
(c) NH3 is more soluble in water but not PH3
(d) Aqueous solution of NH3 has a greater pH value than aqueous solution of PH3.
(ii) Effect on bond angle: The compounds in which hybridization does not take place possess a bond angle of nearly to 90º.
(iii) Existence and non-existence of molecules on the basis of hybridization: For hybridization, the energy difference between the ground state and the excited state must not be very high.
Example: CH4 exists but PH5 does not this is because in CH4, there is an s-p transition taking place, and as the energy difference between s and p orbital is very less whereas in PH5, there is need to be an s-d transition, which is not favorable.
Lesser is the excitation energy better is the hybridization. Excitation can be made easier in 2 ways:
(a) By supplying energy
(b) By reducing the energy gap between orbitals or orbital contraction. This can be done by attaching any electronegative element to the central atom.
For example: In the case of PF3, F being highly electronegative contracts the d orbital of Phosphorus thereby resulting in hybridization while in case of PH3 there is no hybridization. Similarly, in case of PCl3 orbital contraction occurs due to chlorine atoms.
Similarly,
1. PCl5 exist but not PH5
2. 3rd period and onwards elements do not show maximum covalency when attached to lesser electronegative elements like H.
3. Xe forms compounds with F, O like atoms but does not form compounds with H.
Orbital Analysis of Hybridization
knowing the value of i, we can determine hybridization which is equal to spi
Illustration: Determines character in lone pair for PH3 having bond angle 94º.
Solution:
S = 0.06
% of s character in lone pair = 100 – (3 × 6) = 82%
Some Solved Questions
Q.1. The strength of covalent ___________ extent of overlapping of orbitals.
a) may be or may not be related
b) is independent on
c) is dependent on
d) is not related to
Answer: c
Explanation: As per the concept of valence bond theory, the partial merging of atomic orbitals id knowns as overlapping. The extent of overlapping is directly proportional to the strength of the covalent bond, i.e. it is dependent.
Q.2. Which type of bond is present between hydrogens in hydrogen molecule?
a) Sigma bond
b) Pi bond
c) Ionic bond
d) Metallic bond
Answer: a
Explanation: The head-on or end-to-end type of overlapping is present in the sigma bond. A sigma bond is a type of covalent bond. It may also be called an axial overlap. In case of the hydrogen molecule, its s-s overlapping.
Q.3. The pi-bond involves __________
a) axial overlapping
b) side-wise overlapping
c) end to end type of overlapping
d) head-on overlapping
Answer: b
Explanation: A pi-bond is a type of covalent bond in which the internuclear axes of the atoms are parallel to each other and for side-wise overlapping. The bond formed here is perpendicular to the internuclear axes.
Q.4. A __________ overlap doesn’t result in the formation of a bond.
a) positive
b) negative
c) zero
d) rational
Answer: c
Explanation: Zero overlap means that the orbitals don’t overlap at all. When there is no overlapping the bond formation doesn’t occur. As we all know that the extent of overlapping is dependent on the strength of the bond.
Q.5. A positive overlap is same as ________
a) out-phase overlap
b) negative overlap
c) zero overlap
d) in-phase overlap
Answer: d
Explanation: A positive overlap results in bond formation. When 2 p-orbitals are in phase, both the positive lobes overlap, thus creating a positive overlap and result in the bond formation, thus it is called in-phase overlap.
|
50 videos|92 docs|41 tests
|
FAQs on Valence Bond Theory - Chemical Bonding - Inorganic Chemistry
| 1. What is Valence Bond Theory? |  |
| 2. What is the concept of Hybridization of Atomic Orbitals? |  |
| 3. How is Orbital Analysis used in Hybridization? |  |
| 4. Can you provide some examples of Hybridization and its application in chemical bonding? |  |
| 5. Are there any solved questions related to Valence Bond Theory and Hybridization? |  |