Concept Of Atoms And Molecules - Redox Reactions | Physical Chemistry PDF Download
| Table of contents |

|
| Introduction |

|
| 1. The Atom |

|
| Atomic Weight |

|
| 2. The Molecule |

|
| Molecular Weight |

|
| Avogadro's Hypothesis |

|
| 3. Redox Reaction (Oxidation-Reduction Reaction) |

|
Introduction
The Concept of Atoms and Molecules is the cornerstone of chemistry, providing insight into the structure and behavior of matter. Atoms, the smallest units of elements, combine through chemical bonds to form molecules, which are the fundamental units of compounds.
Governed by principles like the laws of conservation of mass and definite proportions, this concept lays the foundation for studying chemical bonding, reactions, and the molecular structure of substances.
1. The Atom
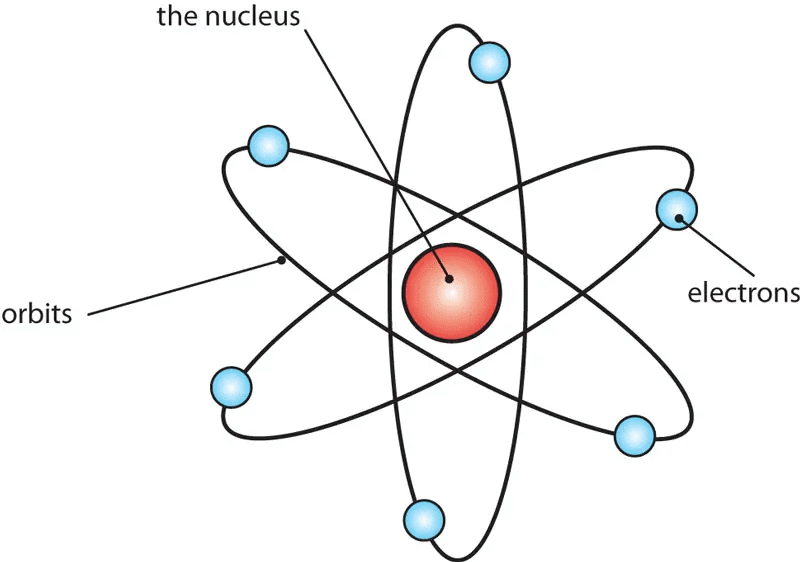
An atom is the smallest unit of matter that retains the properties of an element. It consists of a nucleus (protons and neutrons) surrounded by electrons in specific energy levels.
Atomic Theory: Proposed by John Dalton, stating that matter is composed of indivisible atoms. Atoms of the same element are identical, while those of different elements differ in mass and properties.
Dalton’s Atomic Theory
 Dalton's atomic theory
Dalton's atomic theory
The main postulates of this atomic theory are:
- All matter is made up of tiny, indivisible particles called atoms.
- All atoms of a specific element are identical in mass, size, and other properties. However, atoms of different elements exhibit different properties and vary in mass and size.
- Atoms can neither be created nor destroyed. Furthermore, atoms cannot be divided into smaller particles.
- Atoms of different elements can combine with each other in fixed whole-number ratios in order to form compounds.
- Atoms can be rearranged, combined, or separated in chemical reactions.
Modern Atomic Model: Includes subatomic particles
- Protons (positive charge, found in the nucleus),
- Neutrons (neutral, found in the nucleus),
- Electrons (negative charge, orbiting the nucleus).
- Quantum mechanical model describes electrons in orbitals rather than fixed paths.
 Atom for Carbon
Atom for Carbon
Limitations of Dalton’s Atomic Theory
- Indivisibility of Atoms: Atoms are divisible into subatomic particles (protons, neutrons, electrons).
- Isotopes: Atoms of the same element can have different masses, disproving Dalton’s idea of identical atoms.
- Non-Stoichiometric Compounds: Some compounds (e.g., zinc oxide) do not follow fixed atomic ratios. These are also called Berthollide Compounds, unlike stoichiometric compounds that follow Proust’s law of definite proportions.
- Chemical Bonding: The theory does not explain how atoms bond or the forces involved.
- Mass-Energy Conversion: Part of atomic mass can convert to energy in nuclear reactions.
- Atoms vs. Molecules: No distinction between single atoms and bonded molecules.
Atomic Weight
An atom is so minute that it cannot be detected even with the most powerful microscope, let alone placed on a balance pan and weighed. So there is no question of determining the absolute weight of an atom. So chemists decided to determine the relative masses of atoms (i.e. how many times one atom of an element is heavier than another).
Hydrogen atom was first selected as standard. i.e.
Atomic weight of an element =
- When we state that the atomic weight of chlorine is 35.5, we mean that an atom of chlorine is 35.5 times heavier than an atom of hydrogen.
- It was later felt that the standard for reference for atomic weight may be oxygen, the advantage being that the atomic weights of most other elements became close to whole numbers.
Atomic weight of an element =
The modern reference standard for atomic weights is carbon isotope of mass number 12.
Atomic weight of an element =
On this basis, atomic weight of oxygen 16 was changed to 15.9994.
Atomic weight is called relative atomic mass and denoted by amu (atomic mass unit). The standard for atomic mass is C12.
Atomic weight is not a weight but a number.
Atomic weight is not absolute but relative to the weight of the standard reference element (C12).
Gram atomic weight is atomic weight expressed in grams, but it has a special significance concerning a mole.
Dulong and Petit measured the specific heats of a number of metals and found that the product of specific and the atomic weight is a constant, having an approx. value of 6.4.
Specific heat (cal/g – deg) atomic weight (g/g – atom) ≈ 6.4 (cal/deg.g.atom).
This correlation has been used to ‘correct’ the atomic weights of some elements in the periodic table. Dulong and Petit’s law is applicable only to metals.
2. The Molecule
 Molecule
Molecule
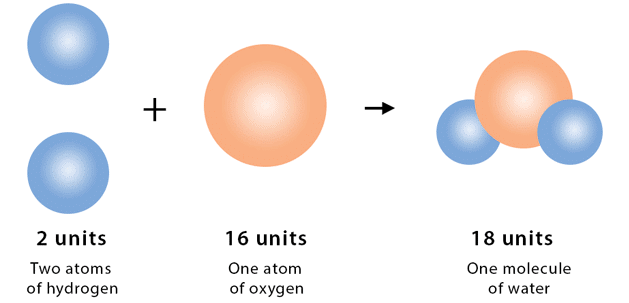
Avogadro (1811) proposed that the fundamental chemical unit is not a single atom but a molecule, which is a cluster of atoms held together and existing as a unit. A molecule is the smallest particle of an element or compound that can exist freely and retain all its properties.
Example - Sulphur Dioxide Molecule: A molecule of sulphur dioxide (SO₂) consists of1 atom of sulphur, 2 atoms of oxygen
When split, it separates into individual atoms, but the properties of sulphur dioxide are retained only in the molecule. Therefore, the molecule is the smallest independent unit of the compound.
Molecules of Compounds: A compound molecule must contain at least two different types of atoms (e.g., H₂O, CO₂).
Molecules of Elements: The term "molecule" also applies to the smallest particle of an element that can exist freely.
For example, hydrogen molecules (H₂) contain 2 atoms. Splitting a hydrogen molecule into individual atoms changes its properties. Nascent hydrogen (atomic hydrogen) is a more powerful reducing agent than molecular hydrogen.
Types of Molecular Structures:
- Diatomic molecules: Molecules of gases like hydrogen (H₂), oxygen (O₂), nitrogen (N₂), and chlorine (Cl₂) have 2 atoms.
- Monoatomic molecules: Noble gases like helium (He), neon (Ne), and argon (Ar) exist as single atoms.
- Tetratomic molecules: Phosphorus (P₄) contains 4 atoms in each molecule.
- Octatomic molecules: Sulphur (S₈) contains 8 atoms in each molecule.
Atomicity: The number of atoms in a molecule of an element is called its atomicity:
- Hydrogen (H₂) → Atomicity = 2
- Sulphur (S₈) → Atomicity = 8
This concept helps explain the structure, properties, and behavior of substances at the molecular level.
Molecular Weight
It is the number of times a molecule is heavier than 1/12 of an atom of C-12.
Molecular Weight =
- Molecular weight is a number, not a weight. It represents the relative mass of a molecule compared to 1/12th the mass of a carbon-12 atom.
Molecular weight is relative, not an absolute measurement.
When molecular weight is expressed in grams, it is called gram-molecular weight.
The molecular weight is calculated by adding the atomic weights of all the atoms in a molecule.
- Examples:
Oxygen (O₂): (2 × 16) = 32
Carbon Dioxide (CO₂): [12 + (2 × 16)] = 44
Sulphuric Acid (H₂SO₄): [(2 × 1) + (1 × 32) + (4 × 16)] = 98 - Molecular weight is now referred to as relative molecular mass.
Avogadro's Hypothesis
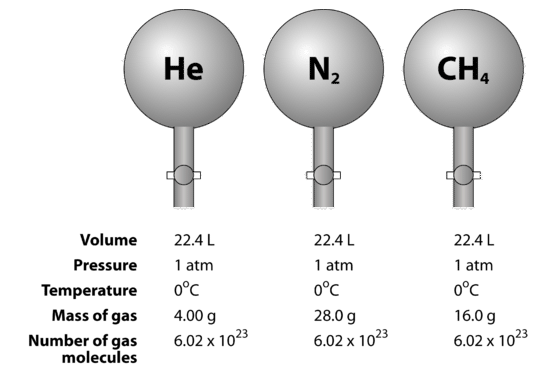 Avogadro HypothesisIt states that equal volumes of gases at the same temperature and pressure contain equal number of molecules. It means that 1m of hydrogen, oxygen, ammonia, or a mixture of gases taken at the same temperature and pressure contains the same number of molecules.
Avogadro HypothesisIt states that equal volumes of gases at the same temperature and pressure contain equal number of molecules. It means that 1m of hydrogen, oxygen, ammonia, or a mixture of gases taken at the same temperature and pressure contains the same number of molecules.Application of Avogadro’s hypothesis:
Determination of Molar Volume of Gases:
Avogadro's hypothesis allows us to determine that 1 mole of any ideal gas, at standard temperature and pressure (STP: 0°C and 1 atm), occupies a volume of 22.4 liters. This is because equal volumes of gases, at the same temperature and pressure, contain the same number of molecules.Calculation of the Number of Molecules in a Given Volume of Gas:
By knowing the volume of a gas and the conditions (temperature and pressure), we can calculate the number of molecules in that gas using Avogadro's number.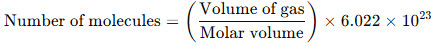
Molecular Mass Determination:
Avogadro’s hypothesis helps in determining the molecular mass of gases. By knowing the volume of a gas sample and its molar volume, we can find the number of moles and calculate the molecular mass.Understanding Stoichiometry in Gas Reactions:
The hypothesis aids in understanding and predicting the ratios in which gases react. For example, in reactions involving gases, the volume ratios of reactants and products are directly proportional to the number of molecules, as explained by Avogadro's hypothesis.Avogadro's Constant and the Concept of Mole:
Avogadro’s hypothesis led to the definition of the mole as a unit of measurement for the amount of substance. One mole of any substance contains 6.022 × 10²³ molecules, helping in the transition from macroscopic quantities to molecular-level understanding in chemistry.Ideal Gas Law:
The relationship described by Avogadro’s hypothesis is a fundamental component of the Ideal Gas Law , which is used to predict the behavior of gases under different conditions of pressure, volume, and temperature.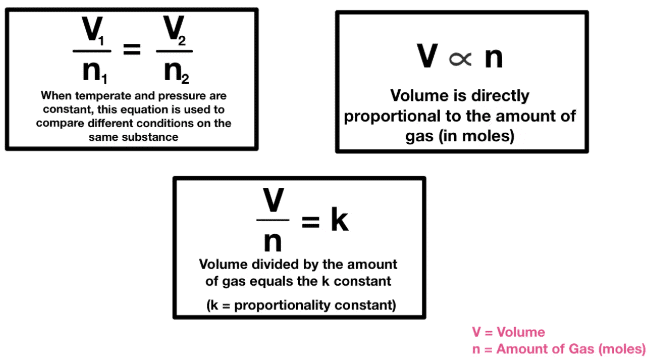
Electrochemical Applications:
Avogadro's hypothesis is used in electrochemical reactions, such as the determination of the amount of substance produced or consumed in an electrolysis reaction. It helps in understanding how much material is involved in such processes at the molecular level.
1. To establish the relationship between molecular weight and vapour density of a gas:
The relative density or vapour density of a gas =
Vapour density of a gas =
So the vapour density of a gas is defined as the ratio of the weight of a certain volume of the gas to the weight of the same volume of hydrogen at the same temperature and pressure.
Vapour density of a gas =
Let ‘V’ litres of gas contain ‘n’ molecules.
Vapour Density of a gas =
Vapour Density of a gas =
Thus, Molecular weight of the gas = 2 x Vapour Density of the gas.
2. Gram-molecular volume or Molar volume:
Molecular weight of a gas =
Molecular weight of a gas =
Gram-molecular weight of a gas = (2/0.089)weight of 1L of the gas at STP
Gram-molecular weight of a gas = 22.4 weight of 1L of the gas at STP = weight of 22.4L of the gas at STP.
This establishes that gram-molecular weight of any gas ( or vapour ) occupies the same volume of 22.4L at STP. The volume occupies by a gram-molecular weight of any gas is called molar volume and its value is 22.4 at STP.
3. Redox Reaction (Oxidation-Reduction Reaction)
A redox reaction is a type of chemical reaction that involves the transfer of electrons between two species. It stands for oxidation (loss of electrons) and reduction (gain of electrons).
Key Concepts:
- Oxidation: The process in which an atom, ion, or molecule loses electrons. It increases the oxidation state of the species.
Example: In the reaction of sodium with chlorine to form NaCl, sodium loses an electron and is oxidized: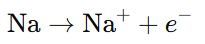
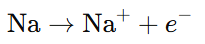
- Reduction: The process in which an atom, ion, or molecule gains electrons. It decreases the oxidation state of the species.
Example: Chlorine gains an electron to form chloride ion:
Oxidizing and Reducing Agents:
- Oxidizing agent: A substance that gains electrons (is reduced) in the reaction.
- Reducing agent: A substance that loses electrons (is oxidized) in the reaction.
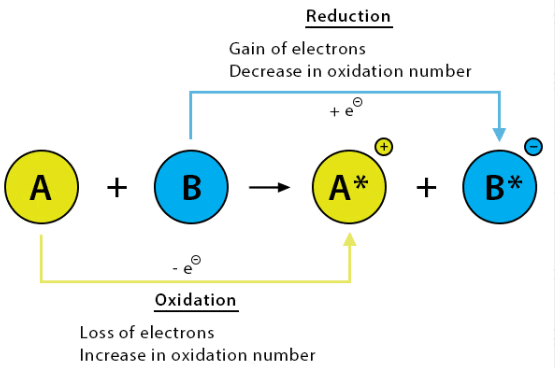
Example of Redox Reaction:
Consider the reaction between hydrogen and oxygen to form water:
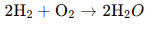
- Hydrogen (H₂) is oxidized (loses electrons) to form H⁺ ions.
- Oxygen (O₂) is reduced (gains electrons) to form O²⁻ ions.
In this case:
- Hydrogen acts as the reducing agent (it loses electrons).
- Oxygen acts as the oxidizing agent (it gains electrons).
Redox reactions are important in a variety of processes, including respiration, combustion, corrosion, and in electrochemical cells like batteries.
|
84 videos|142 docs|67 tests
|
FAQs on Concept Of Atoms And Molecules - Redox Reactions - Physical Chemistry
| 1. What is the concept of atoms and molecules? |  |
| 2. What are redox reactions? |  |
| 3. How do atoms and molecules relate to redox reactions? |  |
| 4. What is an oxidation state in the context of redox reactions? |  |
| 5. How can redox reactions be balanced? |  |
















