JEE Advanced (Single Correct MCQs): Structure of Atom - JEE MCQ
30 Questions MCQ Test - JEE Advanced (Single Correct MCQs): Structure of Atom
The number of neutrons in dipositive zinc ion with mass number 70 is (1979)
Rutherford’s experiment on scattering of a-particles showed for the first time that the atom has (1981 - 1 Mark)
Any p-orbital can accommodate upto (1983 - 1 Mark)
The principal quantum number of an atom is related to the (1983 - 1 Mark)
Rutherford’s scattering experiment is related to the size of the (1983 - 1 Mark)
The increasing order (lowest first) for the values of e/m (charge/mass) for electron (e), proton (p), neutron (n) and alpha particle (α) is : (1984 - 1 Mark)
Correct set of four quantum numbers for the valence (outermost) electron of rubidium (Z = 37) is : (1984 - 1 Mark)
Which electronic level would allow the hydrogen atom to absorb a photon but not to emit a photon? (1984 - 1 Mark)
Bohr model can explain : (1985 - 1 Mark)
The radius of an atomic nucleus is of the order of : (1985 - 1 Mark)
Electromagnetic radiation with maximum wavelength is : (1985 - 1 Mark)
Rutherford’s alpha particle scattering experiment eventually led to the conclusion that : (1986 - 1 Mark)
Which on e of th e following sets of quantum numbers represents an impossible arrangement? (1986 - 1 Mark)
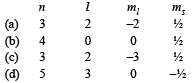
The ratio of the energy of a photon of 2000Å wavelength radiation to that of 4000Å radiation is : (1986 - 1 Mark)
The triad of nuclei that is isotonic is (1988 - 1 Mark)
The wavelength of a spectral line for an electronic transition is inversely related to : (1988 - 1 Mark)
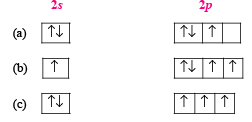
The orbital diagram in which the Aufbau principle is violated is : (1988 - 1 Mark)

The outermost electronic configuration of the most electronegative element is (1988 - 1 Mark)
The correct ground state electronic configuration of chromium atom is : (1989 - 1 Mark)
The correct set of quantum numbers for the unpaired electron of chlorine atom is : (1989 - 1 Mark)
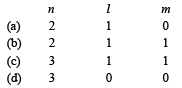
Which of the following does not characterise X-rays? (1992 - 1 Mark)
Which of the following relates to photons both as wave motion and as a stream of particles? (1992 - 1 Mark)
The orbital angular momentum of an electron in 2s orbital is: (1996 - 1 Mark)
For a d-electron, the orbital angular momentum is (1997 - 1 Mark)
The electron s, iden tified by quantum numbers n and l, (i) n = 4, l = 1, (ii) n = 4, l = 0, (iii) n = 3, l = 2, and (iv) n = 3, l = 1 can be placed in order of increasing energy, from the lowest to highest, as (1999 - 2 Marks)
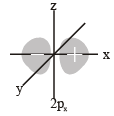
The number of nodal planes in a px orbital is (2000S)
The electronic configuration of an element is 1s2, 2s2 2p6, 3s2 3p6 3d5, 4s1. This represents its (2000S)
The wavelength associated with a golf ball weighing 200 g and moving at a speed of 5 m/h is of the order (2001S)
The quantum numbers +1/2 and –1/2 for the electron spin represent (2001S)



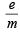 for neutron
for neutron 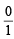 = 0; α-particle =
= 0; α-particle = 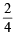 = 0.5;
= 0.5;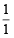 = 1; electron =
= 1; electron = 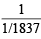 = 1837
= 1837
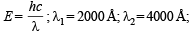
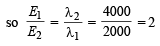
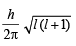
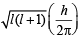
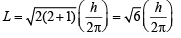
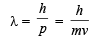
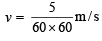
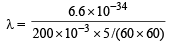 = 2.38 × 10–30 m
= 2.38 × 10–30 m










