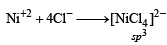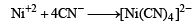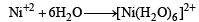JEE Advanced (Single Correct MCQs): Chemical Bonding & Molecular Structure- 2 - JEE MCQ
26 Questions MCQ Test - JEE Advanced (Single Correct MCQs): Chemical Bonding & Molecular Structure- 2
Number of paired electrons in O2 molecule is : (1995S)
Among the following species, identify the isostructural pairs. NF3, NO3- , BF3, H3O+, HN3 (1996 - 1 Mark)
The number and type of bonds between two carbon atoms in CaC2 are : (1996 - 1 Mark)
Which contains both polar and non-polar bonds?
The critical temperature of water is higher than that of O2 because the H2O molecule has (1997 - 1 Mark)
Which one of the following compounds has sp2 hydridization? (1997 - 1 Mark)
The geometry and the type of hybrid orbital present about the central atom in BF3 is (1998 - 2 Marks)
The correct order of increasing C — O bond length of CO, CO2-3, CO2, is (1999 - 2 Marks)
The geometry of H2S and its dipole moment are (1999 - 2 Marks)
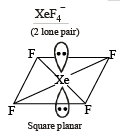
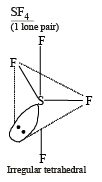
Molecular shapes of SF4, CF4 and XeF4 are (2000S)
The hybridisation of atomic orbitals of nitrogen in NO+2, NO3- and NH+4 are (2000S)
The common features among the species CN–, CO and NO+ are (2001S)
The correct order of hybridization of the central atom in the following species NH3, [PtCl4]2 –, PCl5 and BCl3 is (2001S)
Specify the coordination geometry around and hybridisation of N and B atoms in a 1 : 1 complex of BF3 and NH3
Identify the least stable ion amongst the following : (2002S)
Which of the following molecular species has unpaired electron(s) ? (2002S)
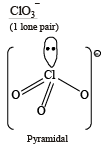
Which of the following are isoelectronic and isostructural?NO3–, CO32–, ClO3–, SO3 (2003S)
According to molecular orbital theory which of the following statement about the magnetic character and bond order is correct regarding O+2 (2004S)
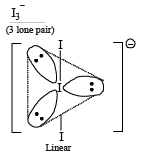
Which species has the maximum number of lone pair of electrons on the central atom? (2005S)
Among the following, the paramagnetic compound is (2007)
The species having bond order different from that in CO is (2007)
Assuming that Hund’s rule is violated, the bond order and magnetic nature of the diatomic molecule B2 is (2010)
The species having pyramidal shape is : (2010)
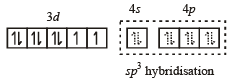
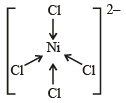
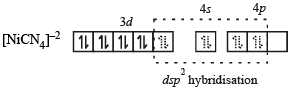
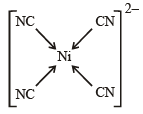
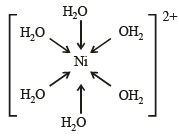
Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl– , CN– and H2O, respectively, are (2011)
Assuming 2s-2p mixing is NOT operative, the paramagnetic species among the following is (JEE Adv. 2014)
The geometries of the ammonia complexes of Ni2+, Pt2+ and Zn2+ respectively, are (JEE Adv. 2016)


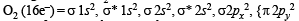
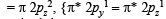 i.e., 2 unpaired and 14 paired electrons.
i.e., 2 unpaired and 14 paired electrons.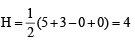
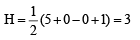
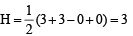
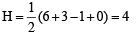

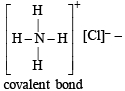 It has ionic and non-polar
It has ionic and non-polar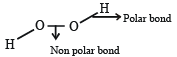
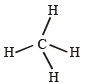
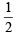 (V + M – C + A)
(V + M – C + A)
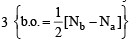
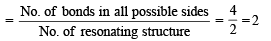
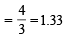
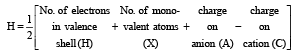
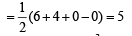

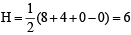
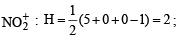
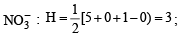
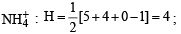
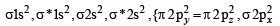
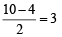
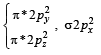
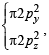
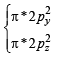
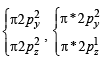
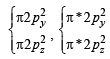
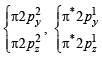
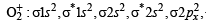
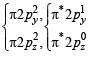
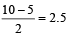
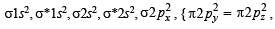
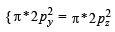
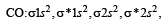
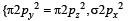
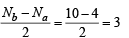
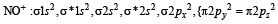
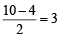
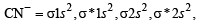
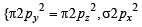
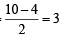
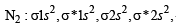
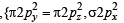
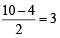
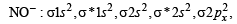
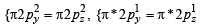
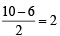
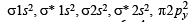
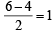 ,B2 will be diamagnetic.
,B2 will be diamagnetic.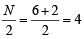 . It has 1 lone pair.
. It has 1 lone pair.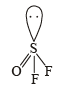 (Shape is trigonal pyramidal)
(Shape is trigonal pyramidal)