Test: JEE Main 35 Year PYQs- Chemical Bonding & Molecular Structure - JEE MCQ
30 Questions MCQ Test - Test: JEE Main 35 Year PYQs- Chemical Bonding & Molecular Structure
In which of the following species the interatomic bond angle is 109° 28’? [2002]
Which of the following are arranged in an increasing order of their bond strengths? [2002]
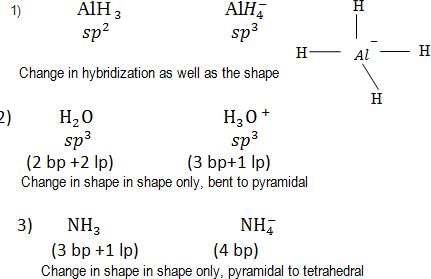
Hybridisation of the underline atom changes in: [2002]
An ether is more volatile than an alcohol having the same molecular formula. This is due to [2003]
Which one of the following pairs of molecules will have permanent dipole moments for both members ? [2003]
Which one of the following compounds has the smallest bond angle in its molecule ? [2003]
The pair of species having identical shapes for molecules of both species is [2003]
The correct order of bond angles (smallest first) in H2S, NH3, BF3 and SiH4 is [2004]
The bond order in NO is 2.5 while that in NO+ is 3. Which of the following statements is true for these two species ? [2004]
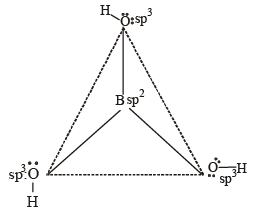
The states of hybridization of boron and oxygen atoms in boric acid (H3BO3) are respectively [2004]
Which one of the following has the regular tetr ahedral structure ? [2004]
(Atomic nos. : B = 5, S = 16, Ni =28, Xe = 54)
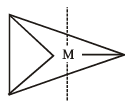
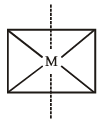
The maximum number of 90º angles between bond pair-bond pair of electrons is observed in [2004]
Lattice energy of an ionic compound depends upon
Which of the following molecules/ions does not contain unpaired electrons? [2006]
In which of the following molecules/ions are all the bonds not equal? [2006]
The decreasing values of bond angles from NH3 (106º) to SbH3 (101º) down group-15 of the periodic table is due to [2006]
Which of the following species exhibits the diamagnetic behaviour ? [2007]
The charge/size ratio of a cation determines its polarizing power. Which one of the following sequences represents the increasing order of the polarizing power of the cationic species, K+, Ca2+, Mg2+, Be2+? [2007]
In which of the following ionization processes, the bond order has increased and the magnetic behaviour has changed? [2007]
Which of the following hydrogen bonds is the strongest? [2007]
Which one of the following pairs of species have the same bond order? [2008]
The bond dissociation energy of B – F in BF3 is 646 kJ mol–1 whereas that of C – F in CF4 is 515 kJ mol–1. The correct reason for higher B – F bond dissociation energy as compared to that of C – F is [2008]
Using MO theory, predict which of the following species has the shortest bond length? [2008]
Among the following the maximum covalent character is shown by the compound [2011]
The hybridization of orbitals of N atom in NO3– , NO2+ and NH4+ are respectively : [2011]
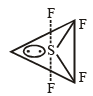
The structure of IF7 is [2011]
Ortho-Nitrophenol is less soluble in water than p- and mNitrophenols because : [2012]
In which of the following pairs the two species are not isostructural ? [2012]
Which one of the following molecules is expected to exhibit diamagnetic behaviour ? [JEE M 2013]
Which of the following is the wrong statement ? [JEE M 2013]


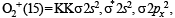
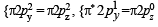
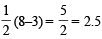
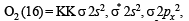
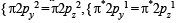
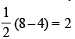
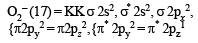
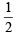 (8 – 5) = 1.5
(8 – 5) = 1.5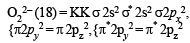
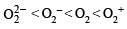

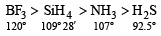
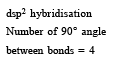
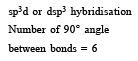
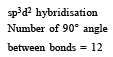
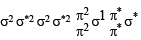
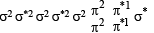
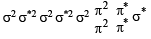
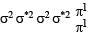
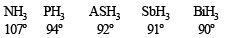
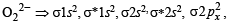
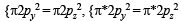
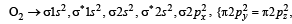
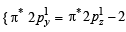 unpaired electrons
unpaired electrons
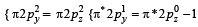 unpaired electron
unpaired electron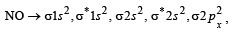
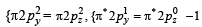 unpaired electron
unpaired electron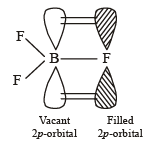
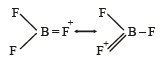
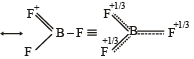
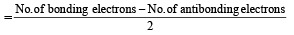
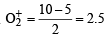
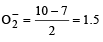
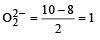
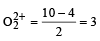
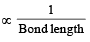
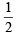 [Number of valence electrons on central atom +No. of monovalent atom altached to it + negative charge if any – positive charge if any]
[Number of valence electrons on central atom +No. of monovalent atom altached to it + negative charge if any – positive charge if any]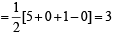
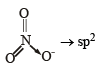
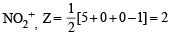
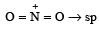
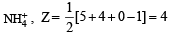
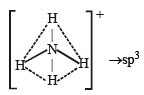
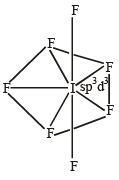
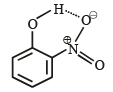
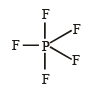
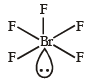
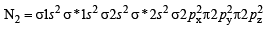
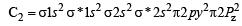
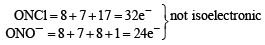
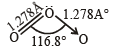 The central atom is sp2 hybridized with one lone pair.
The central atom is sp2 hybridized with one lone pair.















