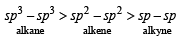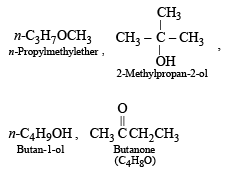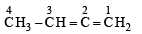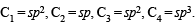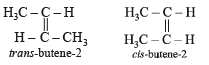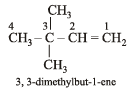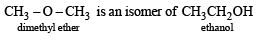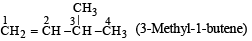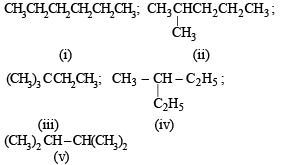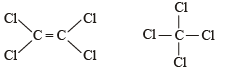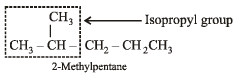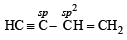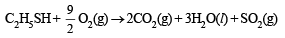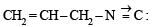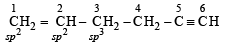JEE Advanced (Single Correct MCQs): Organic Chemistry - Some Basic Principles & Technique - JEE MCQ
30 Questions MCQ Test - JEE Advanced (Single Correct MCQs): Organic Chemistry - Some Basic Principles & Technique
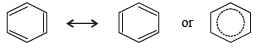
The bond order of in dividual carbon -carbon bonds in benzene is
Molecule in which the distance between the two adjacent carbon atoms is largest is
The compound which is not isomeric with diethyl ether is
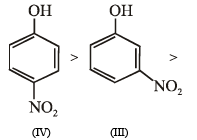
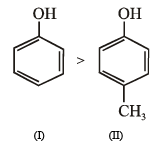
Among the following, the compound that can be most readily sulphonated is
The compound 1, 2-butadiene has
Which of the following compounds will exhibit cis-trans (geometrical) isomerism?
The IUPAC name of the compound having the formula is:
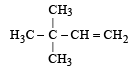
An isomer of ethanol is :
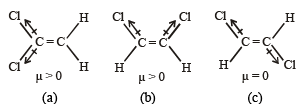
Out of the following compounds, which will have a zero dipole moment?
The bond between carbon atom (1) and carbon atom (2) in compound 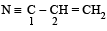 involves the hybrids as
involves the hybrids as
The IUPAC name of the compound
CH2 = CH – CH (CH3)2 is
The number of isomers of C6H14 is
The Cl—C—Cl angle in 1,1,2,2-tetrachloroethene and tetrachloromethane respectively will be about
The compound which has one isopropyl group is :
The C–H bond distance is the longest in :
The number of sigma and pi-bonds in 1-butene-3-yne are :
In CH3CH2OH, the bond that undergoes heterolytic cleavage most readily is
The compound which gives the most stable carbonium ion on dehydration is :
The hybridization of carbon atoms in C–C single bond of HC ≡ C – CH = CH2 is
The products of combustion of an aliphatic thiol (RSH) at 298 K are
Isomers which can be inter converted th rough rotation around a single bond are
The structure 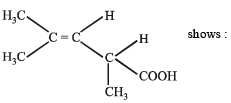
Allyl isocyanide has :
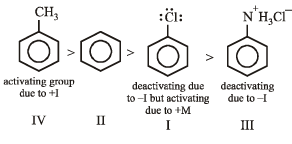
Arrange in order of decreasing trend towards SE reactions :
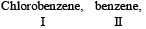
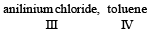
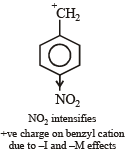
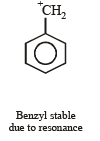
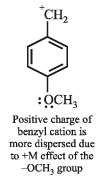
Most stable carbonium ion is :
In the following compounds,
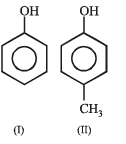
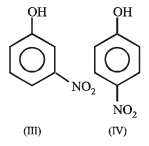
The order of acidity is :
Arrange the following compounds in order of increasing dipole moment.
Toluene (I)
m-dichlorobenzene (II)
o-dichlorobenzene (III)
p-dichlorobenzene (IV)
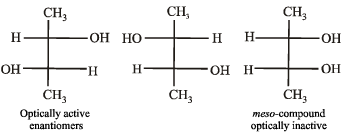
How many optically active stereoisomers are possible for butane-2, 3-diol?
In the compound CH2 = CH–CH2–CH2–C ≡ CH, the C2–C3 bond is of the type,
The optically active tartaric acid is named as D – (+) – tartaric acid because it has a positive