Test: JEE Main 35 Year PYQs- The s-Block Elements - JEE MCQ
24 Questions MCQ Test - Test: JEE Main 35 Year PYQs- The s-Block Elements
Read the following statement and explanation and answer as per the options given below :
Statement : The alkali metals can form ionic hydrides which contain the hydride ion H–.
Explanation : The alkali metals have low electronegativity; their hydrides conduct electricity when fused and liberate hydrogen at the anode.
This question contains STATEMENT-1 (Assertion) and STATEMENT-2 (Reason) and has 4 choices (a), (b), (c) and (d) out of which ONLY ONE is correct.
STATEMENT-1 : Alkali metals dissolve in liquid ammonia to give blue solutions. because
STATEMENT-2 : Alkali metals is liquid ammonia give solvated species of the type [M(NH3)n]+ (M = alkali metals).
KO2 (potassium super oxide) is used in oxygen cylinders in space and submarines because it
The metallic sodium disolves in liquid ammonia to form a deep blue coloured solution. The deep blue colour is due to formation of:
A metal M readily forms its sulphate MSO4 which is watersoluble. It forms its oxide MO which becomes inert on heating. It forms an insoluble hyroxide M(OH)2 which is soluble in NaOH solution. Then M is
In curing cement plasters water is sprinkled from time to time. This helps in
The substance not likely to contain CaCO3 is
The solubilities of carbonates decrease down the magnesium group due to a decrease in
Which one of the following processes will produce hard water ?
One mole of magnesium nitride on the reaction with an excess of water gives :
Which of the following species is diamagnetic in nature?
Based on lattice energy and other considerations which one of the following alkali metal chlorides is expected to have the highest melting point ?
Which of the following statements in relation to the hydrogen atom is correct ?
The ionic mobility of alkali metal ions in aqueous solution is maximum for
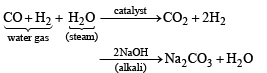
In context with the industrial preparation of hydrogen from water gas (CO + H2), which of the following is the correct statement?
Which of the following on thermal decomposition yields a basic as well as acidic oxide ?
Very pure hydrogen (99.9) can be made by which of the following processes ?
In which of the following reactions H2O2 acts as a reducing agent?
Which one of the following alkaline earth metal sulphates has its hydration enthalpy greater than its lattice enthalpy ?
The molecular formula of a commercial resin used for exchanging ions in water softening is C8H7SO3– Na+ (Mol. wt. 206. What would be the maximum uptake of Ca2+ ions by the resin when expressed in mole per gram resin ?
From the following statements regarding H2O2, choose the incorrect statement :
Which one of the following statements about water is FALSE?
Which of the following atoms has the highest first ionization energy?
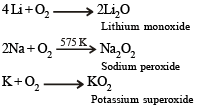
The main oxides formed on combustion of Li, Na and K in excess of air are, respectively:



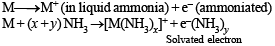
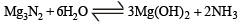
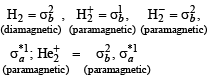
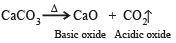
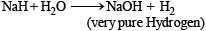
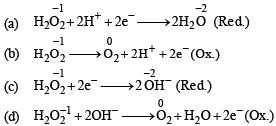
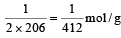 will be maximum uptake
will be maximum uptake