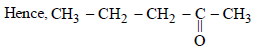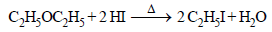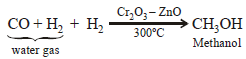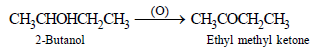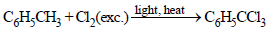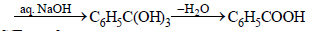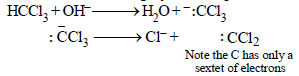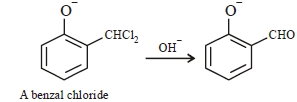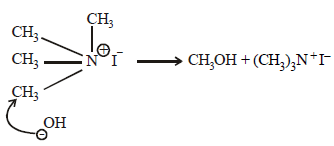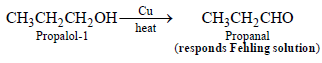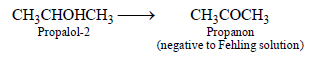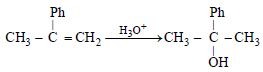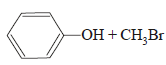JEE Advanced (Single Correct MCQs): Alcohols, Phenols & Ethers - JEE MCQ
20 Questions MCQ Test - JEE Advanced (Single Correct MCQs): Alcohols, Phenols & Ethers
Ethyl alcohol is heated with conc H2SO4 the product formed is
Which of the following is basic
The compound which reacts fastest with Lucas reagent at room temperature is
A compound that gives a positive iodoform test is
Diethyl ether on heating with conc. HI gives two moles of
An industrial method of preparation of methanol is :
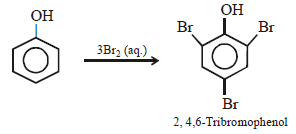
When phenol is treated with excess bromine water, it gives:
HBr reacts fastest with :
Which of the following compounds is oxidised to prepare methylethyl ketone?
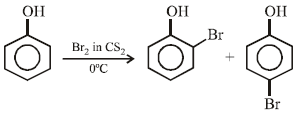
Phenol reacts with bromine in carbon disulphide at low temperature to give:
Chlorination of toluene in the presence of light and heatfollowed by treatment with aqueous NaOH gives:
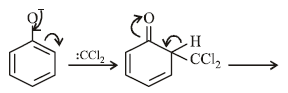
When phenol is reacted with CHCl3 and NaOH followed by acidification, salicyladehyde is obtained. Which of the
following species are involved in the above mentioned reaction as intermediate?
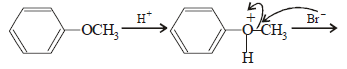
The compound that will react most readily with NaOH to form methanol is
1-Propanol and 2-propanol can be best distinguished by:
If the ethanol is in excess then 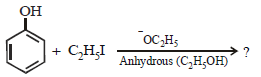
The product of acid catalyzed hydration of 2-phenylpropene is:
The best method to prepare cyclohexene from cyclohexanol is by using:
The increasing order of boiling points of the below mentioned alcohols is :
(I) 1,2-dihydroxybenzene
(II) 1,3-dihydroxybenzene
(III) 1,4-dihydroxybenzene
(IV) Hydroxybenzene
In the reaction 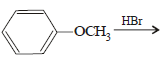 the products are
the products are
For the identification of b-naphthol using dye test, it is necessary to use:




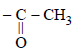 groups show positive iodoform test.
groups show positive iodoform test.