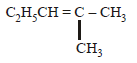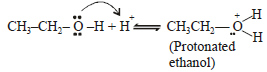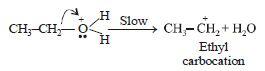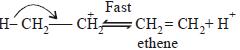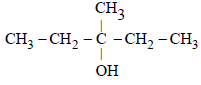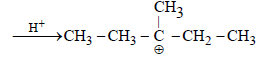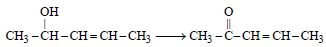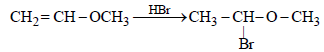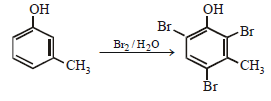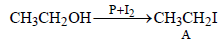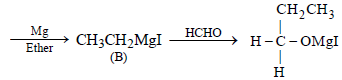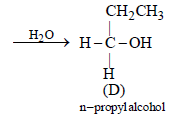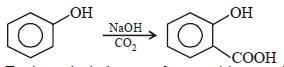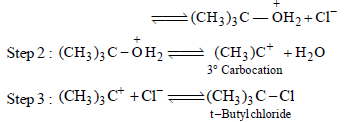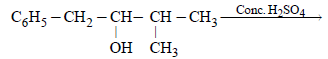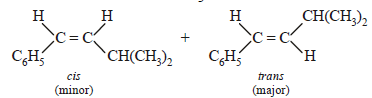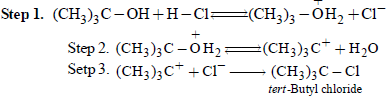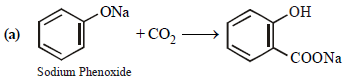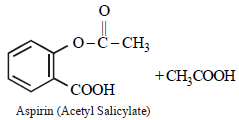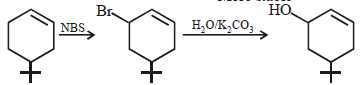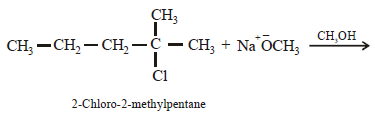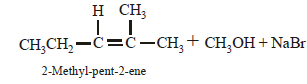Test: JEE Main 35 Year PYQs- Alcohols, Phenols & Ethers - JEE MCQ
20 Questions MCQ Test - Test: JEE Main 35 Year PYQs- Alcohols, Phenols & Ethers
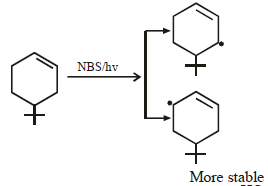
During dehydration of alcohols to alkenes by heating with conc. H2SO4 the initiation step is [2003]
Among the following compounds which can be dehydrated very easily is [2004]
The best reagent to convert pent-3-en-2-ol into pent-3-in-2-one is [2005]
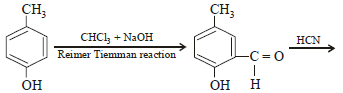
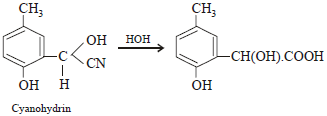
p -cresol reacts with chloroform in alkaline medium to give the compound A which adds hydrogen cyanide to form, the
compound B. The latter on acidic hydrolysis gives chiral carboxylic acid. The structure of the carboxylic acid is
[2005]
HBr reacts with CH2 = CH – OCH3 under anhydrous conditions at room temperature to give [ 2006]
Among the following the one that gives positive iodoform test upon reaction with I2 and NaOH is [ 2006]
The structure of the compound that gives a tribromo derivative on treatment with bromine water is [2006]
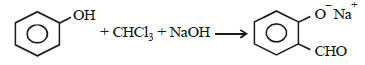
The electrophile involved in the above reaction is
In the following sequence of reactions,
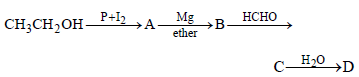
the compound D is [2007
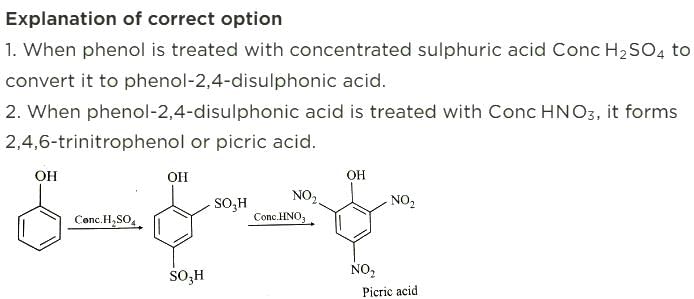
Phenol, when it first reacts with concentrated sulphuric acidand then with concentrated nitric acid, gives [2008]
The major product obtained on interaction of phenol withsodium hydroxide and carbon dioxide is [2009]
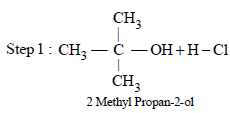
From amongst the following alcohols the one that wouldreact fastest with conc. HCl and anhydrous ZnCl2, is [2010]
The main product of the following reaction is
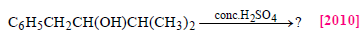
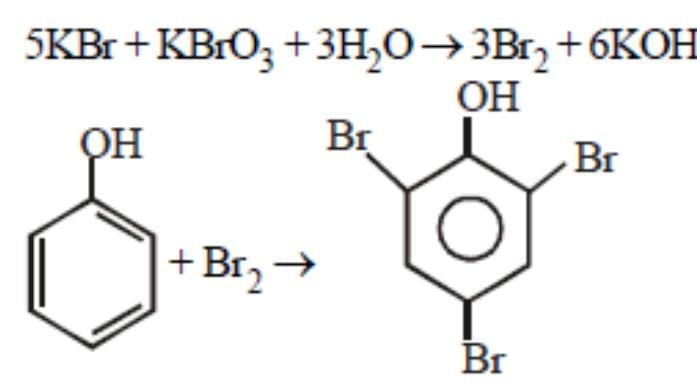
Phenol is heated with a solution of mixture of KBr and KBrO3. The major product obtained in the above reaction is :[2011]
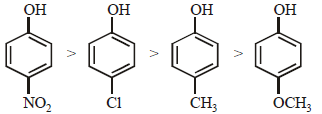
Arrange the following compounds in order of decreasing acidity :
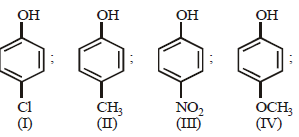
An unknown alcohol is treated with the “Lucas reagent” todetermine whether the alcohol is primary, secondary ortertiary. Which alcohol reacts fastest and by what mechanism:[JEE M 2013]
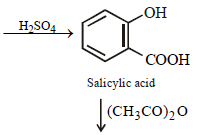
Sodium phenoxide when heated with CO2 under pressure at 125ºC yields a product which on acetylation produces C.
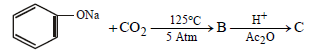
The major product C would be [JEE M 2014]
Thiol group is present in : [JEE M 2016]
The product of the reaction given below is: [JEE M 2016]
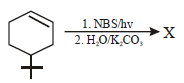
2-chloro-2-methylpentane on reaction with sodium methoxide in methanol yields: [JEE M 2016]
(i)
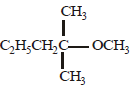
(ii)
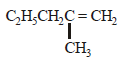
(iii)