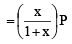Test: JEE Main 35 Year PYQs- Equilibrium - JEE MCQ
30 Questions MCQ Test - Test: JEE Main 35 Year PYQs- Equilibrium
PARAGRAPH 1
When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7 °C was measured for the beaker and its contents (Expt.1).
Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)
Q. Enthalpy of dissociation (in kJ mol–1) of acetic acid obtained from the Expt.2 is
Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)
PARAGRAPH 1
When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7 °C was measured for the beaker and its contents (Expt.1).
Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)
Q. The pH of the solution after Expt. 2 is
Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)
PARAGRAPH 2
Thermal decomposition of gaseous X2 to gaseous X at 298 K takes place according to the following equation:
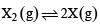
The standard reaction Gibbs energy, ΔrG°, of this reaction is positive. At the start of the reaction, there is one mole of X2 and no X. As the reaction proceeds, the number of moles of X formed is given by β. Thus, βequilibrium is the number of moles of X formed at equilibrium. The reaction is carried out at a constant total pressure of 2 bar. Consider the gases to behave ideally.
(Given R = 0.083 L bar K–1 mol–1)
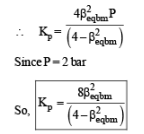
Q. The equilibrium constant Kp for this reaction at 298 K, in terms of βequilibrium, is
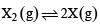
PARAGRAPH 2
Thermal decomposition of gaseous X2 to gaseous X at 298 K takes place according to the following equation:
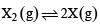
The standard reaction Gibbs energy, ΔrG°, of this reaction is positive. At the start of the reaction, there is one mole of X2 and no X. As the reaction proceeds, the number of moles of X formed is given by β. Thus, βequilibrium is the number of moles of X formed at equilibrium. The reaction is carried out at a constant total pressure of 2 bar. Consider the gases to behave ideally.
(Given R = 0.083 L bar K–1 mol–1)
Q. The INCORRECT statement among the following, for this reaction, is
Each question contains STATEMENT-1 (Assertion) and STATEMENT-2 (Reason). Each question has 5 choices (a), (b), (c) and (d) out of which ONLY ONE is correct.
Q.
Statement -1 The endothermic reactions are favoured at lower temperature and the exothermic reactions are favoured at higher temperature.
Statement -2 When a system in equilibrium is disturbed by changing the temperature, it will tend to adjust itself so as to overcome the effect of change.
Read the following statement and explanation and answer as per the options given below :
Statement -1 HNO3 is a stronger acid than HNO2
Statement -2 In HNO3 there are two nitrogen-to-oxygen bonds whereas in HNO2 there is only one.
Read the following statement and explanation and answer as per the options given below :
Statement -1 For every chemical reaction at equilibrium, standard Gibbs energy of reaction is zero.
Statement -2 At constant temperature and pressure, chemical reactions are spontaneous in the direction of decreasing Gibbs energy.
1 M NaCl and 1 M HCl are present in an aqueous solution.
The solution is
Species acting as both Bronsted acid and base is
Let the solubility of an aqueous solution of Mg(OH)2 be x then its Ksp is
Change in volume of the system does not alter which of the following equilibria?
For the reaction CO (g) + (1/2) O2 (g) = CO2 (g), Kp / Kc is
Which one of the following statements is not true?
The solubility in water of a sparingly soluble salt AB2 is 1.0 × 10–5 mol L–1. Its solubility product number will be
For the reaction equilibrium
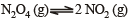
the concentrations of N2O4 and NO2 at equilibrium are 4.8 × 10–2 and 1.2 × 10–2 mol L–1 respectively. The value of Kc for the reaction is
Consider the reaction equilibrium
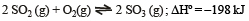
On the basis of Le Chatelier’s principle, the condition favourable for the forward reaction is
When rain is accompanied by a thunderstorm, the collected rain water will have a pH value
What is the equilibrium expression for the reaction 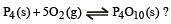
The equilibrium constant for the reaction 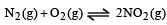 at temperature T is 4×10–4.
at temperature T is 4×10–4.
The value of Kc for the reaction
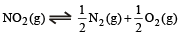 at the same temperature is
at the same temperature is
The molar solubility (in mol L–1) of a sparingly soluble salt MX4 is ‘s’. The corresponding solubility product is Ksp. ‘s’ is given in term of Ksp by the relation :
If α is the degree of dissociation of Na2SO4 , the Vant Hoff’s factor (i) used for calculating the molecular mass is
The solubility product of a salt having general formula MX2 , in water is : 4 × 10 -12. The concentration of M2+ ions in the aqueous solution of the salt is
The exothermic formation of CIF3 is represented by the equation :
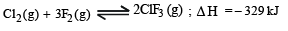
Which of the following will increase the quantity of CIF3 in an equilibrium mixture of Cl2 , F2 and ClF3 ?
For the reaction 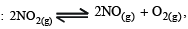
(Kc = 1.8 x 10-6 at 184°C) (R = 0.0831 kJ/ (mol. K))
When Kp and Kc are compared at 184°C, it is found that
Hydrogenion concentration in mol/L in a solution of pH = 5.4 will be :
An amount of solid NH4 HS is placed in a flask already containing ammonia gas at a certain temperature and 0.50 atm pressure. Ammonium hydrogen sulphide decomposes to yield NH3 and H2S gases in the flask. When the decomposition reaction reaches equilibrium, the total pressure in the flask rises to 0.84 atm? The equilibrium constant for NH4HS decomposition at this temperature is
Phosphorus pentachloride dissociates as follows, in a closed reaction vessel
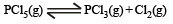
If total pressure at equilibrium of the reaction mixture is P and degree of dissociation of PCl5 is x, the partial pressure of PCl3 will be


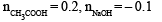
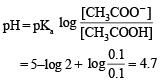

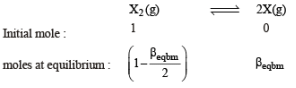

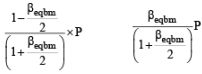
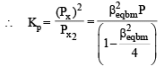
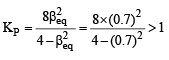
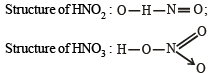
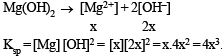
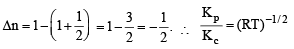
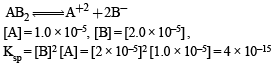
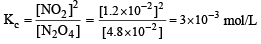
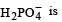
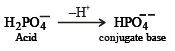
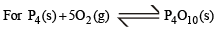
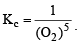 The solids have con centration unity
The solids have con centration unity
 is equal to
is equal to
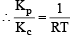
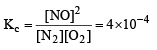
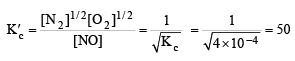
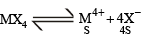
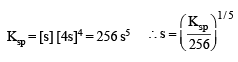
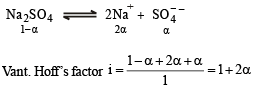
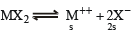
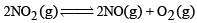
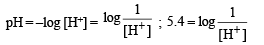
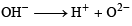 Conjugate base of OH– is O2–
Conjugate base of OH– is O2–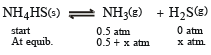
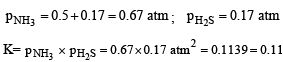
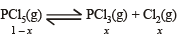
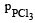 = mole fraction of PCl3 × Total pressure
= mole fraction of PCl3 × Total pressure