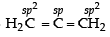JEE Advanced (Single Correct MCQs): Hydrocarbons - JEE MCQ
30 Questions MCQ Test - JEE Advanced (Single Correct MCQs): Hydrocarbons
Which of the following decolourises alkaline KMnO4 solution(1980)
The compound with the highest boiling point is (1982 - 1 Mark)
The maximum number of isomers for an alkene with the molecular formula C4H8 is (1982 - 1 Mark)
When propyne is treated with aqueous H2SO4 in presence of HgSO4 the major product is (1983 - 1 Mark)
Which of the following compounds does not dissolve in conc. H2SO4 even on warming? (1983 - 1 Mark)
Baeyer ’s reagent is : (1984 - 1 Mark)
Acidic hydrogen is present in : (1985 - 1 Mark)
Anti-Markovnikoff addition of HBr is not observed in : (1985 - 1 Mark)
The highest boiling point is expected for : (1986 - 1 Mark)
Which of the following will have least hindered rotation about carbon-carbon bond? (1987 - 1 Mark)
When cyclohexane is poured on water, it floats, because:
The product(s) obtained via oxymercuration (HgSO4 + H2SO4) of 1- butyne would be (1999 - 2 Marks)
Propyne and propene can be distinguished by (2000S)
Which one of the following will react fastest with H2 under catalytic hydrogenation condition ? (2000S)
In the presence of peroxide, hydrogen chloride and hydrogen iodide do not give anti-Markovnikov addition to alkenes because(2001S)
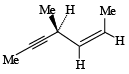
Hydrogenation of the above compound in the presence of poisoned palladium catalyst gives (2001S)
The reaction of propene with HOCl proceeds via the addition of(2001S)
The nodal plane in the π-bond of ethene is located in
Consider the following reaction (2002S)
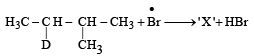
Identify the structure of the major product 'X'
Identify the reagent from the following list which can easily distinguish between 1-butyne and 2-butyne (2002S)
Which of the following is used for the conversion of 2-hexyne into trans-2-hexene ? (2004S)
On monochlorination of 2-methylbutane, the total number of chiral compounds formed is (2004S)
Identify the product, P in the following reaction : CH3 - CH = CH2 + NOCl —→ P (2006 - 3M, –1)
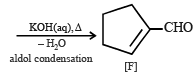
Cyclohexene on ozonolysis followed by reaction with zinc dust and water gives compound E. Compound E on further treatment with aqueous KOH yields compound F.
Compound F is (2007)
The synthesis of 3-octyne is achieved by adding a bromoalkane into a mixture of sodium amide and an alkyne.The bromoalkane and alkyne respectively are (2010)
The bond energy (in kcal mol–1) of a C–C single bond is approximately (2010)
In allene (C3H4), the type(s) of hybridisation of the carbon atoms is (are) : (2012)
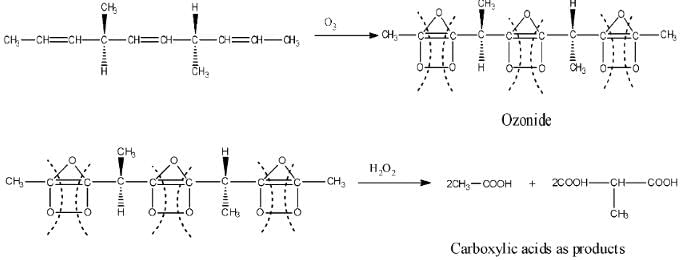
The number of optically active products obtained from the complete ozonolysis of the given compound is : (2012)
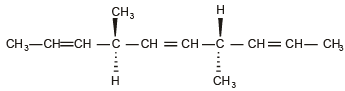



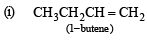
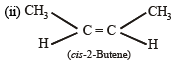
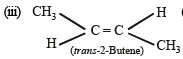
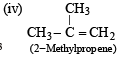
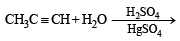
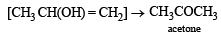
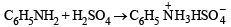
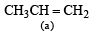
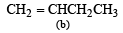
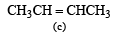
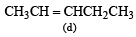
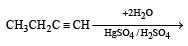
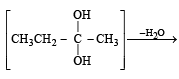
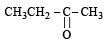
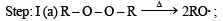
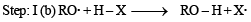
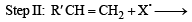
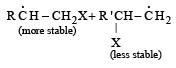
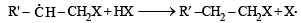
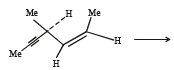
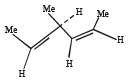
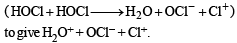
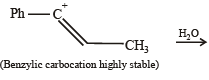
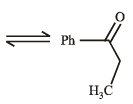
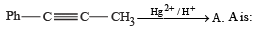 (2003S)
(2003S)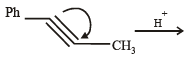
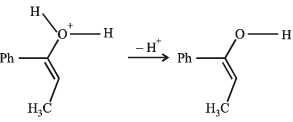
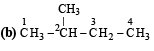
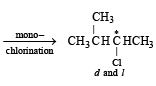
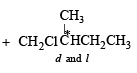
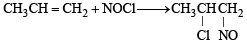
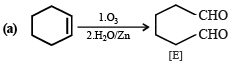
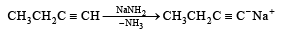
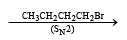
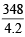 kcal/mol
kcal/mol