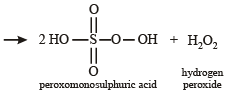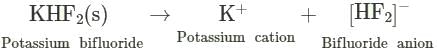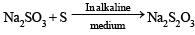JEE Advanced (Single Correct MCQs): The p-Block Elements - JEE MCQ
30 Questions MCQ Test - JEE Advanced (Single Correct MCQs): The p-Block Elements
The reddish brown coloured gas formed when nitric oxide is oxidised by air is
The temporary hardness of water due to calcium carbonate can be removed by adding –
Which of the following is most stable to heat
White P reacts with caustic soda. The products are PH3 and NaH2PO2. This reaction is an example of
A solution of KBr is treated with each of the following.
Which one would liberate bromine
Which of the following is coloured
HBr and HI reduce sulphuric acid, HCl can reduce KMnO4 and HF can reduce
Which of the following statements about anhydrous aluminium chloride is correct?
Moderate electrical conductivity is shown by
Chlorine acts as a bleaching agent only in presence of
Nitrogen dioxide cannot be obtained by heating :
A gas that cannot be collected over water is :
The compound which gives off oxygen on moderate heating is :
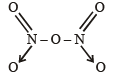
The bonds present in N2O5 are :
Which of the following oxides of nitrogen is a coloured gas?
Amongst the trihalides of nitrogen which one is least basic?
Bromine can be liberated from potassium bromide solution by the action of
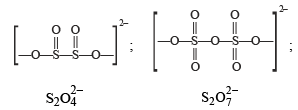
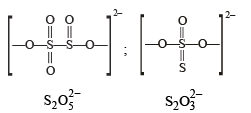
There is no S–S bond in :
In P4O10 each P atom is linked with .......... O atoms
H2SO, cannot be used to prepare HBr from NaBr as it
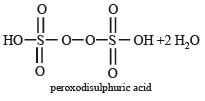
Hydrolysis of one mole of peroxodisulphuric acid produces
Which of the following statements is correct for CsBr3?
KF combines with HF to form KHF2. The compound contains the species.
Sodium thiosulphate is prepared by
Which of the following halides is least stable and has doubtful existence?
Which one of the following oxides is neutral?
Which one of the following species is not a pseudohalide?
One mole of calcium phosphide on reaction with excess water gives


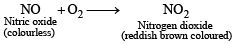
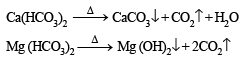

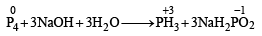
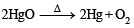
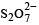 there is an S–O–S bond unlike in other.
there is an S–O–S bond unlike in other.