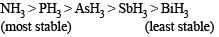Test: JEE Main 35 Year PYQs- The p-Block Elements - JEE MCQ
30 Questions MCQ Test - Test: JEE Main 35 Year PYQs- The p-Block Elements
Alum helps in purifying water by
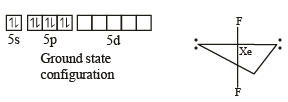
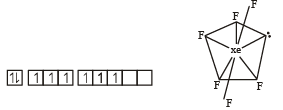
In XeF2, XeF4, XeF6 the number of lone pairs on Xe are respectively
In case of nitrogen, NCl3 is possible but not NCl5 while in case of phosphorous, PCl3 as well as PCl5 are possible. It is due to
Which of the following statements is true?
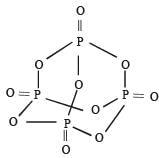
Number of sigma bonds in P4O10 is
Oxidation number of Cl in CaOCl2 (bleaching power) is:
What may be expected to happen when phosphine gas is mixed with chlorine gas ?
Concentrated hydrochlor ic acid when kept in open air sometimes produces a cloud of white fumes. The explanation for it is that
Graphite is a soft solid lubricant extremely difficult to melt. The reason for this anomalous behaviour is that graphite
Which one of the following substan ces has the highest proton affinity ?
For making good quality mirrors, plates of float glass are used. These are obtained by floating molten glass over a liquid metal which does not solidify before glass. The metal used can be
Which among the following factors is the most important in making fluorine the strongest oxidizing halogen ?
Which one of the following statement regarding helium is incorrect ?
Beyllium and aluminium exhibit many properties which are similar. But, the two elements differ in
Aluminium chloride exists as dimer, Al2Cl6 in solid state as well as in solution of non-polar solvents such as benzene.
When dissolved in water, it gives
Excess of KI reacts with CuSO4 solution and then Na2S2O3 solution is added to it. Which of the statements is incorrect for this reaction ?
The number of hydrogen atom(s) attached to phosphorus atom in hypophosphorous acid is
The correct order of the thermal stability of hydrogen halides (H – X) is
Heating an aqueous solution of alumin ium chloride to dryness will give
The structure of diborane (B2H6) contains
Which of the following statements is true?
The increasing order of the first ionization enthalpies of the elements B, P, S and F (Lowest first) is
What products are expected from the disproportionation reaction of hypochlorous acid?
Identify the incorrect statement among the following.
Regular use of the following fertilizers increases the acidity of soil?
Which one of the following is the correct statement?
Which one of the following reactions of xenon compounds is not feasible?
Which of the following statement is wrong?





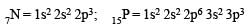

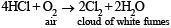
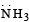 is most basic. Hence has highest proton affinity
is most basic. Hence has highest proton affinity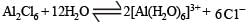
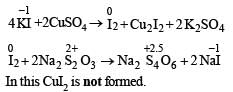

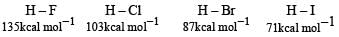

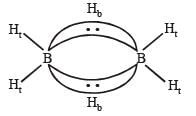
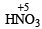 is stronger than
is stronger than 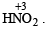 The more the oxidation state of N, the more is the acid character.
The more the oxidation state of N, the more is the acid character.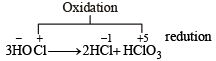
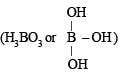 is a lewis acid so (a) is
is a lewis acid so (a) is