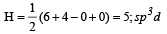31 Year NEET Previous Year Questions: Chemical Bonding & Molecular Structure - 2 - NEET MCQ
30 Questions MCQ Test - 31 Year NEET Previous Year Questions: Chemical Bonding & Molecular Structure - 2
The correct order of the O–O bond length in O2, H2O2 and O3 is [1995, 2005]
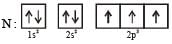
The ground state electronic configuration of valence shell electrons in nitrogen molecule (N2) is written as KK 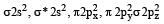 Bond order in nitrogen molecule is [1995]
Bond order in nitrogen molecule is [1995]
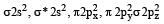 Bond order in nitrogen molecule is [1995]
Bond order in nitrogen molecule is [1995]The correct order of N–O bond lengths in NO, NO2–, NO3– and N2O4 is [1996]
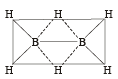
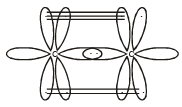
Which of the following compounds has a 3-centre bond?[1996]
The low density of ice compared to water is due to[1997]
The cylindrical shape of an alkyne is due to the fact that it has [1997]
N2 and O2 are converted into monocations, N2+ and O2+ respectively. Which of the following statements is wrong ? [1997]
The AsF5 molecule is trigonal bipyramidal. The hybrid orbitals used by the As atom for bonding are [1997]
The number of anti-bonding electron pairs in O2-2 molecular ion on the basis of molecular orbital theory is, (Atomic number of O is 8) [1998]
Among the following which one is not paramagnetic? [Atomic numbers : Be = 4, Ne = 10, As = 33, Cl = 17] [1998]
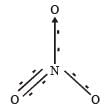
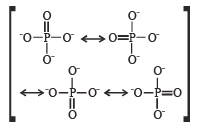
In PO43– ion, the formal charge on each oxygen atom and P—O bond order respectively are
Which of the following molecules is planar?
The dipole moments of diatomic molecules AB and CD are 10.41D and 10.27 D, respectively while their bond distances are 2.82 and 2.67 Å, respectively. This indicates that [1999]
Which one of the following arrangements represents the increasing bond orders of the given species? [1999]
Which one of the following has the pyramidal shape?[1999]
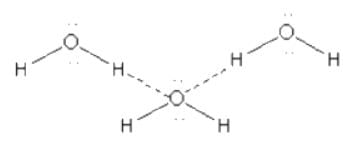
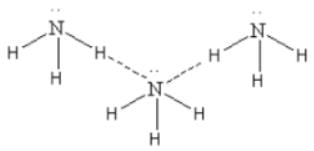
Which one of the following molecules will form a linear polymeric structure due to hydrogen bonding? [2000]
Among the following the electron deficient compound is : [2000]
The relationship between the dissociation energy of N2 and N2+ is : [2000]
Cation and anion combines in a crystal to form following type of compound. [2000]
Among the following ions the pπ–dπ overlap could be present in [2000]
In which of the following the bond angle is maximum? [2001]
Which of the following two are isostructural?
Main axis of a diatomic molecule is z, molecular orbital px and py overlap to form which of the following orbital? [2001]
In X — H --- Y, X and Y both are electronegative elements
Which of the following has pπ – dπ bonding?
In NO3– ion number of bond pair and lone pair of electrons on nitrogen atom respectively are
[2002]
Which of the following statements is not correct for sigma and pi-bonds formed between two carbon atoms? [2003]
Among the following the pair in which the two species are not isostructural is [2004]
In a regular octahedral molecule, MX6 the number of X - M - X bonds at 180° is [2004]
H2O is polar, whereas BeF2 is not. It is because [2004]


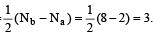

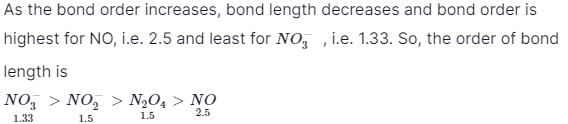
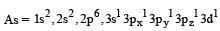
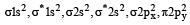
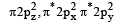
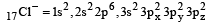
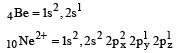
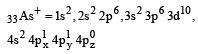
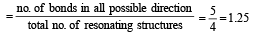

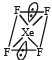 Square planar shape.
Square planar shape.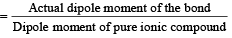
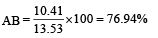
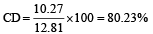
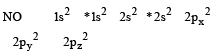
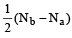
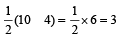
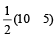
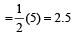
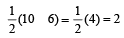
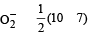
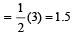
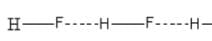
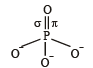
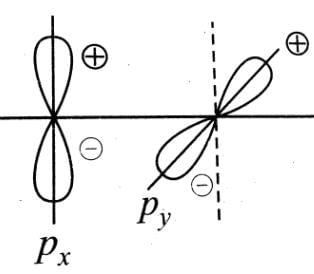
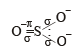
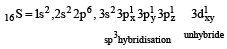
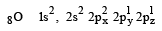
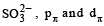 orbitals ar e involved for pπ - dπbonding.
orbitals ar e involved for pπ - dπbonding.