31 Year NEET Previous Year Questions: Thermodynamics - 2 - NEET MCQ
30 Questions MCQ Test - 31 Year NEET Previous Year Questions: Thermodynamics - 2
If ΔH is the change in enthalpy and ΔE, the change in internal energy accompanying a gaseous reaction, then [1990]
Equal volumes of molar hydrochloric acid and sulphuric acid are neutralized by dil. NaOH solution and x kcal and y kcal of heat are liberated respectively. Which of the following is true ?
During isothermal expansion of an ideal gas, its
A chemical reaction will be spontaneous if it is accompanied by a decrease of [1994]
Consider the following reaction occurring in an automobile [1994]
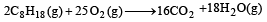
the sign of ΔH, ΔS and ΔG would be
Standard Gibb’s free energy change for isomerization reaction [1995] cis-2 pentene  trans-2-pentene is – 3.67 kJ/mol at 400 K. If more trans-2 pentene is added to the reaction vessel, then
trans-2-pentene is – 3.67 kJ/mol at 400 K. If more trans-2 pentene is added to the reaction vessel, then
If enthalpies of formation of C2H4(g) , CO2(g) and H2O(l) at 25°C and 1atm pressure are 52, – 394 and – 286 kJ/mol respectively, the change in ethalpy is equal to [1995]
The correct relationship between free energy and equilibrium con stant K of a reaction is [1996]
According to the third law of thermodynamics which one of the following quantities for a perfectly crystalline solid is zero at absolute zero?
Given the following entropy values (in J K–1 mol–1) at 298 K and 1 atm :H2(g) : 130.6, Cl2(g) : 223.0, HCl (g) : 186.7.The entropy change (in J K–1 mol– 1) for the reaction H2(g) + Cl 2(g) —→ 2 HCl(g) is [1996]
Hydrogen has an ionisation energy of 1311 kJ mol–1 and for chlorine it is 1256 kJ mol–1.Hydrogen forms H+ (aq) ions but chlorine does not form Cl+ (aq) ions because [1996]
Given that 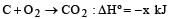
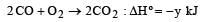
the enthalpy of formation of carbon monoxide will be [1997]
Identify the correct statement regarding entropy: [1998]
One mole of an ideal gas at 300 K is expanded isothermally from an initial volume of 1 litre to 10 litres. The ΔE for this process is (R =2 cal. mol–1 K–1)[1998]
For a cyclic process, which of the following is not true? [1999]
Adiabatice xpansions of an ideal gas is accompanied by [1999]
For a reaction in which all reactants and products are liquids, which one of the following equations is most applicable ? [1999]
The entropy change in the fusion of one mole of a solid melting at 27ºC (Latent heat of fusion, 2930 J mol–1) is : [2000]
The factor of ΔG values is important in metallurgy. The ΔG values for the following reactions at 800ºC are given as :
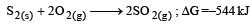
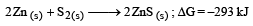
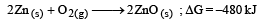
Then ΔG for the reaction :
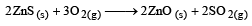 will be :
will be :
The values of heat of formation of SO2 and SO3 are –298.2 kJ and –98.2 kJ. The heat of formation of the reaction 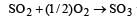 will be [2000]
will be [2000]
For a hypothetical reaction A → B , the activation energies for forward and backward reactions are 19 kJ/mole and 9 kJ/mole respectively. The heat of reaction is [2000]
Enthalpy of 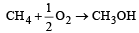 is negative. If enthalpy of combustion of CH4 and CH3OH are x and y respectively, then which relation is correct [2001]
is negative. If enthalpy of combustion of CH4 and CH3OH are x and y respectively, then which relation is correct [2001]
When 1 mol of a gas is heated at constant volume, temperature is raised from 298 to 308 K. If heat supplied to the gas is 500 J, then which statement is correct ? [2001]
What is the enth alpy chan ge for, 2H2O2(l) → 2 H2O(l) + O2(g) if h eat of formation of H2O2 (l) and H2O (l) are –188 and – 286 kJ/mol respectively? [2001]
In a closed insulated container, a liquid is stirred with a paddle to increase the temperature, which of the following is true? [2002]
2 mole of an ideal gas at 27ºC temperature is expanded reversibly from 2 lit to 20 lit. Find the entropy change (R = 2 cal/mol K) [2002]
Heat of combustion ΔHº for C (s), H2(g) and CH4(g) are –94, –68 and –213 kcal/mol, then ΔHº for C(s) + 2H2(g) → CH4(g) is [2002]
Unit of entropy is [2002]
The molar heat capacity of water at constant pressure is 75 JK–1 mol–1. When 1kJ of heat is supplied to 100 g of water, which is free to expand, the increase in temperature of water is [2003]


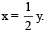

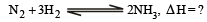
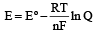
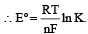 ................(i)
................(i)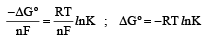
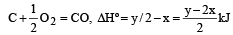
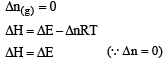

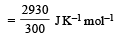
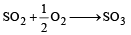
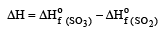
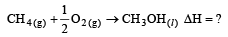
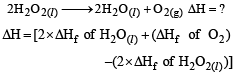
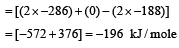
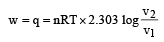
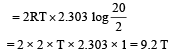
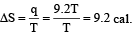
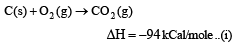
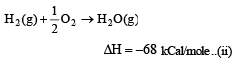
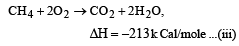
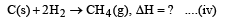
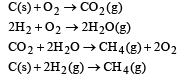
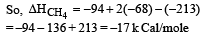
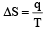
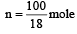 Q = 1000J ΔT = ?
Q = 1000J ΔT = ?