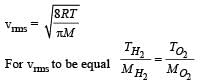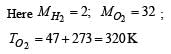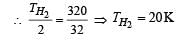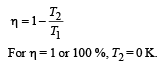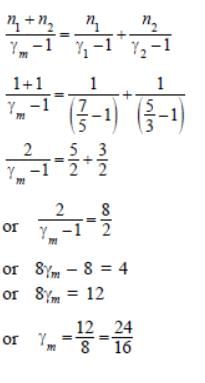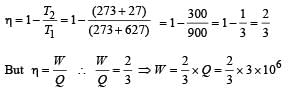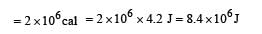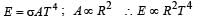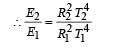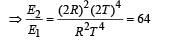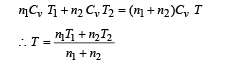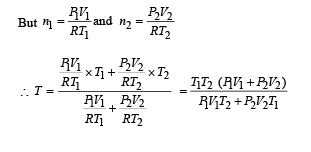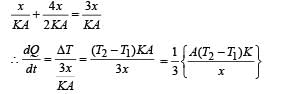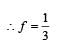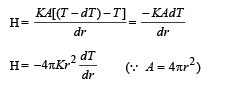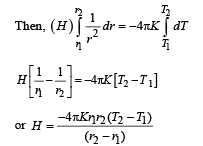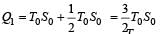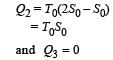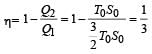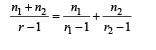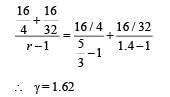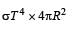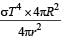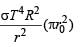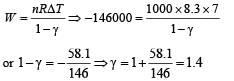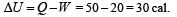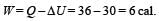Test: JEE Main 35 Year PYQs- Heat & Thermodynamics - JEE MCQ
30 Questions MCQ Test - Test: JEE Main 35 Year PYQs- Heat & Thermodynamics
Heat given to a body which raises its temperature by 1°C is
Infrared radiation is detected by
Which of the following is more close to a black body?
Cooking gas containers are kept in a lorry moving with uniform speed. The temperature of the gas molecules inside will
If mass-energy equivalence is taken into account, when water is cooled to form ice, the mass of water should
At what temperature is the r.m.s velocity of a hydrogen molecule equal to that of an oxygen molecule at 47°C?
Even Carnot engine cannot give 100% efficiency because we cannot
1 mole of a gas with γ = 7/5 is mixed with 1 mole of a gas with γ = 5/3, then the value of g for the resulting mixture is
Two spheres of the same material have radii 1 m and 4 m and temperatures 4000 K and 2000 K respectively. The ratio of the energy radiated per second by the first sphere to that by the second is
“Heat cannot by itself flow from a body at lower temperature to a body at higher temperature” is a statement or consequen ce of
During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its absolute temperature.
The ratio CP /CV for the gas is
Which of the following parameters does not characterize the thermodynamic state of matter?
A Carnot engine takes 3 *10 6 cal. of heat from a reservoir at 627o C , and gives it to a sink at 27 o C . The work done by the engine is
The earth radiates in the infra-red region of the spectrum. The spectrum is correctly given by
According to Newton’s law of cooling, the rate of cooling of a body is proportional to ( Δθ)n , wher e Δθ is the difference of the temperature of the body and the surroundings, and n is equal to
One mole of ideal monatomic gas ( γ = 5 / 3) is mixed with one mole of diatomic gas (γ = 7 / 5) . What is g for the mixture? γ Denotes the ratio of specific heat at constant pressure, to that at constant volume
If the temperature of the sun were to increase from T to 2T and its radius from R to 2R, then the ratio of the radiant energy received on earth to what it was previously will be
Which of the following statements is correct for any thermodynamic system ?
Two thermally insulated vessels 1 and 2 are filled with air at temperatures (T1 ,T2 ), volume (V1 ,V2) and pressure ( P1 ,P2) respectively. If the valve joining the two vessels is opened, the temperature inside the vessel at equilibrium will be
The temperature of the two outer surfaces of a composite slab, consisting of two materials having coefficients of thermal conductivity K and 2K and thickness x and 4x, respectively, are T2 and T1 (T2 >T1) . The rate of heat transfer through the slab, in a steady state is with f equal to
Which of the following is incorrect regarding the first law of thermodynamics?
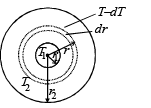
The figure shows a system of two concentric spheres of radii r1 and r2 are kept at temperatures T1 and T2, respectively. The radial rate of flow of heat in a substance between the two concentric spheres is proportional to
A system goes from A to B via two processes I and II as shown in figure. If ΔU1 and ΔU2 are the changes in internal energies in the processes I and II respectively, then
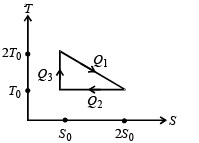
The temperature-entropy diagram of a reversible engine cycle is given in the figure. Its efficiency is
A gaseous mixture consists of 16 g of helium and 16 g of oxygen. The ratio Cp/Cv of the mixture is
Assuming the Sun to be a spherical body of radius R at a temperature of TK, evaluate the total radiant powerd incident of Earth at a distance r from the Sun
where r0 is the radius of the Earth and σ is Stefan's constant.
Two rigid boxes containing different ideal gases are placed on a table. Box A contains one mole of nitrogen at temperature T0, while Box contains one mole of helium at temperature 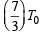 . The boxes are then put into thermal contact with each other, and heat flows between them until the gases reach a common final temperature (ignore the heat capacity of boxes). Then, the final temperature of the gases, Tf in terms of T0 is
. The boxes are then put into thermal contact with each other, and heat flows between them until the gases reach a common final temperature (ignore the heat capacity of boxes). Then, the final temperature of the gases, Tf in terms of T0 is
The work of 146 kJ is performed in order to compress one kilo mole of gas adiabatically and in this process the temperature of the gas increases by 7°C. The gas is (R = 8.3 J mol–1 K–1)
When a system is taken from state i to state f along the path iaf, it is found that Q =50 cal and W = 20 cal. Along the path ibf Q = 36 cal. W along the path ibf is