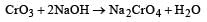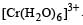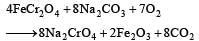32 Year NEET Previous Year Questions: The d & f-Block Elements - 1 - NEET MCQ
30 Questions MCQ Test - 32 Year NEET Previous Year Questions: The d & f-Block Elements - 1
Which of the following metals corrodes readily in moist air ? [1980]
Which one of the following is an ore of silver ? [1983]
The oxidation state of Cr in K2Cr2O7 is[1988]
Prussian blue is formed when [1989]
Photographic films and plates have an essential ingredient of [1989]
Nitriding is the process of surface hardening of steel by treating it in an atmosphere of [1989]
While extracting an element form its ore, the ore is ground and leached with dil. potassium cyanide solution to form the soluble product potassium argento cyanide. The element is [1989]
The electronic configurations of four elements are given below. Which element does not belong to the same family as others ? [1989]
The composition of ‘golden spangles’ is [1990]
A blue colouration is not obtained when [1989]
The electronic configuration of Cu (atomic number 29) is [1991]
Cinnabar is an ore of [1991]
The transition elements have a general electronic configuration [1991]
When CuSO4 is electrolysed using platinum electrodes, [1993]
The most durable metal plating on iron to protect against corrosion is [1994]
The common oxidation states of Ti are [1994]
When (NH4)2 Cr2O7 is heated, the gas evolved is[1994]
Actinides [1994]
Among the lanthanides the one obtained by synthetic method is [1994]
Stainless steel contains iron and [1995]
Cuprous compounds such as CuCl, CuCN and CuSCN are the only salts stable in water due to
K2Cr2O7 on heating with aqueous NaOH gives [1997]
The lanthanide contraction is responsible for the fact that [1997] (Atomic numbers : Zr = 40, Y = 39, Nb = 41, Hf = 72, Zn = 30)
CrO3 dissolves in aqueous NaOH to give [1997]
The electroniccon figuration of gadolinium (Atomic number 64) is [1997]
Which one of the following elements constitutes a major impurity in pig iron ? [1998]
Which one of the following elements shows maximum number of different oxidation states in its compounds? [1998]
Which one of the following ionic species will impart colour to an aqueous solution? [1998]
On heating chromite (FeCr2O4) with Na2CO3 in air, which of the following product is obtained? [1999]
The addition of excess of aqueous HNO3 to a solution containing [Cu(NH3)4]2+ produces [1999]



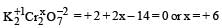
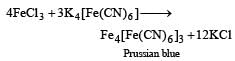
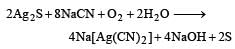
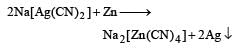
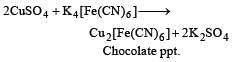
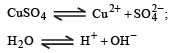
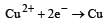
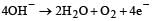
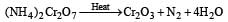
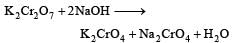
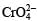 ion is obtained.
ion is obtained.