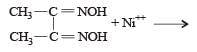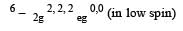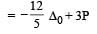31 Year NEET Previous Year Questions: Coordination Compounds - NEET MCQ
30 Questions MCQ Test - 31 Year NEET Previous Year Questions: Coordination Compounds
Which of the following is considered to be an anticancer species ?
Which of the following coordination compounds would exhibit optical isomerism? [2004]
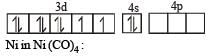
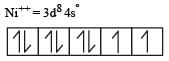
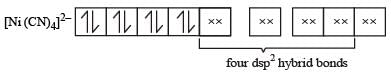
Among [ Ni(CO)4],[ Ni(CN) 4]2- ,[ NiCl4]2- species, the hybridization states of the Ni atom are, respectively(At. No. of Ni = 28) [2004]
CN– is a strong field ligand. This is due to the fact that [2004]
Considering H2O as a weak field ligand, the number of unpaired electrons in [Mn(H2O)6]2+ will be (At. no. of Mn = 25) [2004]
Which of the following does not have a metalcarbon bond? [2004]
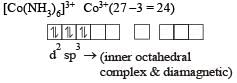
Which one of the following is an inner or bital complex as well as diamagnetic in behaviour? (Atomic number: Zn = 30, Cr = 24, Co = 27, Ni = 28) [2 00 5]
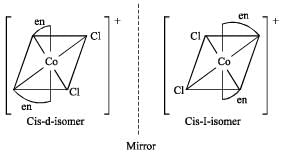
Which one of the following is expected to exhibit optical isomerism? (en = eth ylen ediamine) [2 00 5]
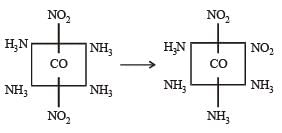
[Co(NH3)4 (NO2)2] Cl exhibits [2006]
[Cr(H2O)6]Cl3 (at no. of Cr = 24) has a magnetic moment of 3.83 B. M. The correct distribution of 3d electrons in the Chromium of the complex is
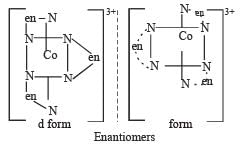
Which of the following will give a pair of enantiomorphs? [2007]
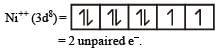
The d electron configurations of Cr2+, Mn2+, Fe2+ and Ni2+ are 3d4, 3d5, 3d6 and 3d8 respectively. Which one of the following aqua complexes will exhibit the minimum paramagnetic behaviour? [2007] (At. No. Cr = 24, Mn = 25, Fe = 26, Ni = 28)
Which of the following complexes exhibits the highest paramagnetic behaviour ? [2008] where gly = glycine, en = ethylenediamine and bpy = bipyridyl moities) (At.nosTi = 22, V = 23, Fe = 26, Co = 27)
In which of the following coordination entities the magnitude Δ0 (CFSE in octahedral field) will be maximum? [2008]
Which of the following does not show optical isomerism? [2009]
Which of the following complex ions is expected to absorb visible light? [2009] (At. no. Zn = 30, Sc = 21, Ti = 22, Cr = 24)
Which of the following complex ion is not expected to absorb visible light ? [2010]
Crystal field stabilization energy for high spin 4 octahedral complex is: [2010]
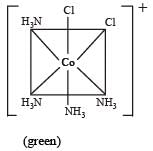
The existence of two different coloured complexes with the composition of [Co(NH3)4 Cl2]+ is due to : [2010]
Which one of the following complexes is not expected to exhibit isomerism? [2010]
Of the following complex ions, which is diamagnetic in nature ? [2011]
Th e complexes [Co(NH3)6] [Cr (CN)6] and [Cr(NH3)6] [Co(CN)6] are the examples of which type of isomerism? [2011]
The complex, [Pt(Py)(NH3)BrCl] will have how many geometrical isomers ? [2011]
The d-electron configurations of Cr 2+, Mn2+, Fe2+ and Co2+ are d4, d5, d6 and d7, respectively.Which one of the following will exhibit minimum paramagnetic behaviour? [2011] (At, nos. Cr = 24, Mn = 25, Fe = 26, Co = 27)
Which of the following carbonyls will have the strongest C – O bond ? [2011 M]
Which of the following complex compounds will exhibit highest paramagnetic behaviour? [2011M] (At. No. : Ti = 22, Cr = 24, Co = 27, Zn = 30)
Which one of the following is an outer orbital complex and exhibits paramagnetic behaviour ? [2012]
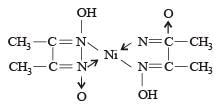
Red precipitate is obtained when ethanol solution of dimethylglyoxime is added to ammoniacal Ni(II). Which of the following statements is not true ? [2012 M]
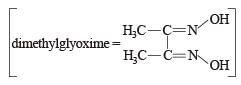
Low spin complex of d6-cation in an octahedral field will have the following energy : [2012 M] (Δ0= Crystal Field Splitting Energy in an octahedral field, P = Electron pairing energy)
A magnetic moment of 1.73 BM will be shown by one among the following : [NEET 2013]



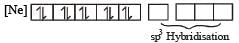
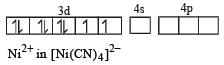
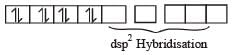
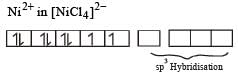
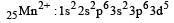
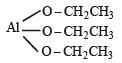



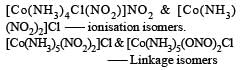
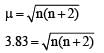
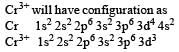
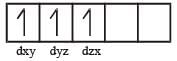
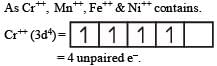


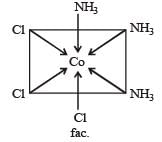
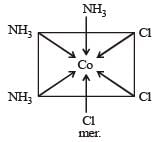

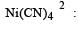 Number of unpaired electrons = 0
Number of unpaired electrons = 0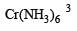 : Number of unpaired electrons = 3
: Number of unpaired electrons = 3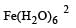 : Number of unpaired electrons = 4
: Number of unpaired electrons = 4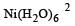 : Number of unpaired electrons = 2
: Number of unpaired electrons = 2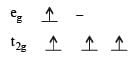
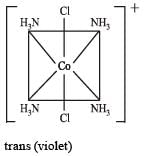

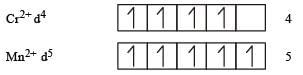
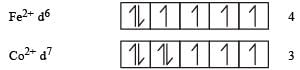
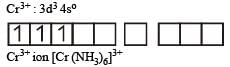

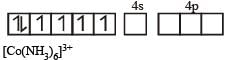
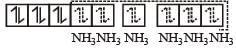
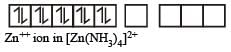
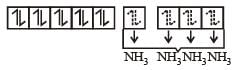
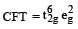 therefore, hybridisation is sp3d2 & complex is paramagnetic.
therefore, hybridisation is sp3d2 & complex is paramagnetic.