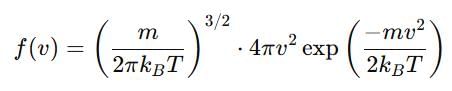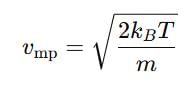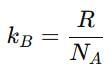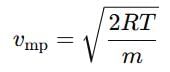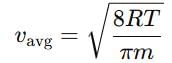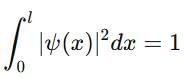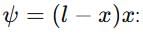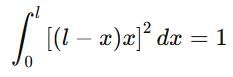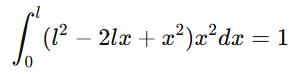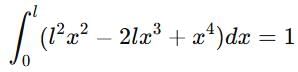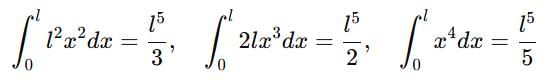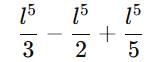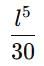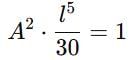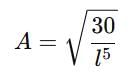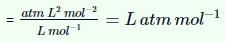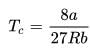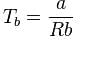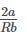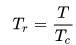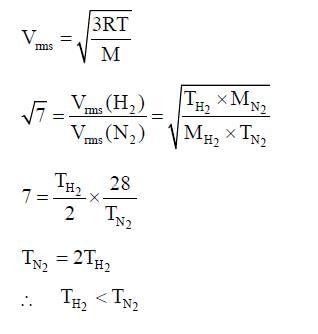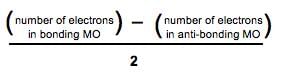IIT JAM Chemistry - MCQ Test 1 - Chemistry MCQ
30 Questions MCQ Test - IIT JAM Chemistry - MCQ Test 1
Distribution of molecules with velocity is represented by curve as shown:
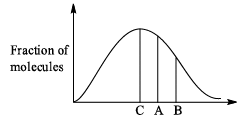
Velocity at point A is:

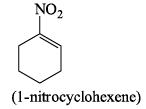
Which of the following drawings is not a resonance structure of 1 -nitrocyclohexene:

If a particle in the box of length 'l' has wavelength, ψ = (l -x)x. (x is only variable) What is its normalization constant?
What is the correct increasing order of bond lengths of bond indicated in following compund?

The most stable canonical structure among all of above is:
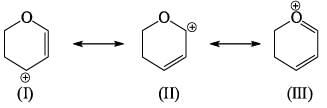
Compare relative stability of the following structures:

Strength of following bases decrease in the order?
(I) Br- (II) F- (III) NH2 (IV) CH3-
The ratio of van der Waal’s constant a and b, 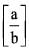 has the dimensions of:
has the dimensions of:
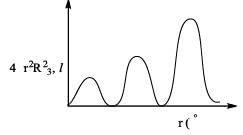
Find the corresponding subshell utilizing the information from graph.
Of the following acids, the one that is strongest is:
Lattice energy (numerical value) of chloride of alkali metals is in order:
Which of the following is not resonating structure of each other?
Examine the following resonating structures of formic acid for their individual stability and then answer the question given below:


Which of the following arrangements gives the correct order of decreasing stability of the above-mentioned resonance contributors?
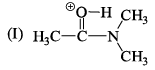
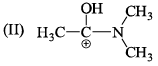
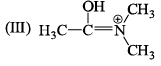
The correct stability order of the given resonating structures is:
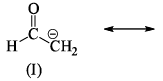
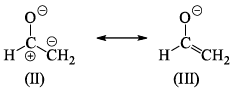
The correct order of stability for the given canonical structures is:
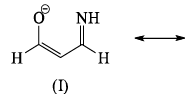
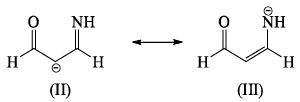
The correct order of stability among the following canonical structure is:
Match column I with column II and select the correct answers:



The table indicates the value of van-der Waal’s constant ‘a’. The gas which can be liquefied most easily is:
rms velocity of hydrogen is √7 times the rms velocity of nitrogen. If T is the temperature of gas,then:
Copper containing alloy weighing 0.3175g dissolved in an acid, and an excess of KI is added.
Estimation of copper in alloy is based on following reactions:
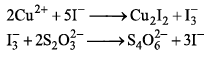
If a particle has linear momentum 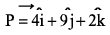 at position
at position 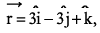 , then its angular momentum is:
, then its angular momentum is:
Consider two molecules A & B.

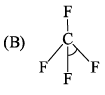
∠α = ∠HCH; ∠β = ∠FCF
Which of following is true?
Predict the shape of IF4+ molecule using VSEPR theory:
Which of the following is true for ionization energy:
Arrange the following in decreasing order of their bond angles: NH3, H2O , CH4
The ground state electronic configuration of valence shell electrons in a molecule A2 is written as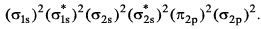 • Hence bond order is_____________________.
• Hence bond order is_____________________.