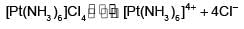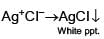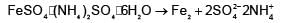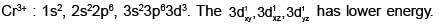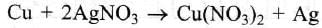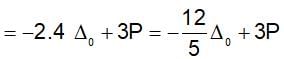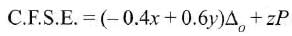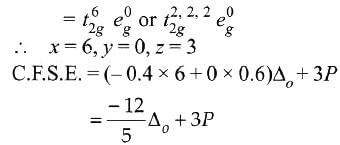Coordination Compounds - Class 12 MCQ
30 Questions MCQ Test - Coordination Compounds
The EAN of platinum in potassium hexachloroplatinate (IV) is:
Which will give a white precipitate with AgNO3 in aqueous solution?
The number of ions formed on dissolving one molecule of FeSO4.(NH4)2SO4.6H2O in water is:
Which possesses tetrahedral shape (sp3 - hybridization of central atom?
Which complex has square planar shape & dsp2- hybridization?
The number of precipitable halide ions in [Pt(NH3)CI2Br]CI is:
Which statement about coordination number of metal ion is true?
The correct name of the compound [Cu(NH3)4](NO3)2, according to IUPAC system is:
The formula of a carbonyl complex of (CO)n CO(CO)n, in which there is a single covalent Co-Co bond is?
Of the following complexes, the one with the largest value of the crystal field splitting is:
In which complex is the transition metal in zero oxidation state?
From the stability constant (hypothetical values) given below, predict which is the strongest ligand?
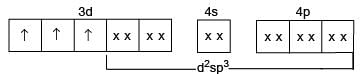
Which of the following complex species do not involve d2sp3-hybridization?
Which of the following compounds would exhibit coordination isomerism?
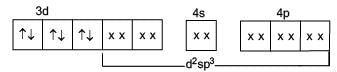
[Cr(H2O6]CI3 (at. no. of Cr=24) has a magnetic moment of 3.83 BM. The correct distribution of 3d-electrons in the chromium of the complex:
The hypothetical complex chloro diaquatriammine cobalt(III) chloride can be represented as:
In the silver plating of Cu, K[Ag(CN)2] is used instead of AgNO3. The reason is:
Low spin complex of d6-cation in an octahedral field will have the following energy: