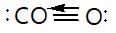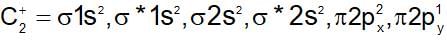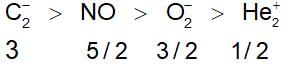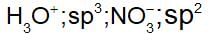Chemical Bonding - Grade 10 MCQ
30 Questions MCQ Test - Chemical Bonding
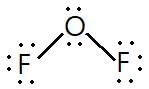
In OF2, number of bond pairs and lone pairs of electrons are respectively:
Which are true statements among the following?
(I) PH5 and BiCI5 does not exist
(II) pπ-dπ bonds are present in SO2
(III) Electrons travel with speed of light
(IV) SeF4 and CH4 has same shape
(V) I+3 has bent geometry
(II) pπ-dπ bonds are present in SO2
(III) Electrons travel with speed of light
(IV) SeF4 and CH4 has same shape
(V) I+3 has bent geometry
Increasing order (lower first) of size of the various hybridised orbitals is:
Which of the following statement regarding carbon monoxide is correct?
The incorrect statements regarding bonding molecular orbitals because:
The cyanide ion, CN- and N2 are isoelectronic. But in contrast to CN- , N2 is chemically insert, because of :
H2O has a net dipole moment, while BeF2 has zero dipole moment, because:
Four diatomic species are listed below in different sequence. Which of these represents the correct order of their increasing bond order?
Some of the properties of the two species,NO-3 and H3O+ are describe below. Which one of them is correct?
The total number of π-bond electrons in the following structure is:
Give the correct order of initials True (T) or False (F) for the following statements.
(I) sp3 hybrid orbitals are 900 to one other. (II) Adjacent sp3d2 hybrid orbitals are at 900 to one another. (III) sp2 hybrid orbitals are at 1200 to one other (IV) Bond order of N-O bond in NO-3 is 4/3.
Which of the following statements is not correct regarding NO2 molecule?