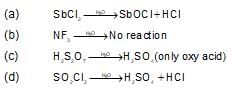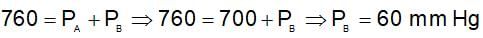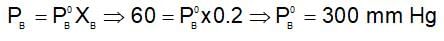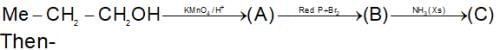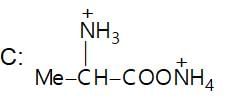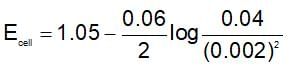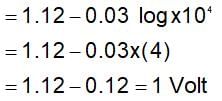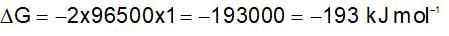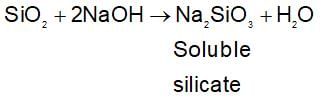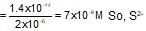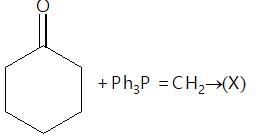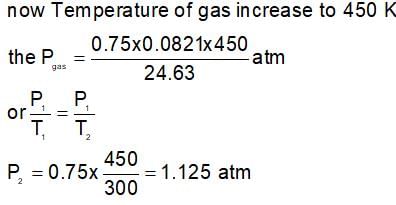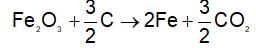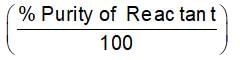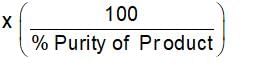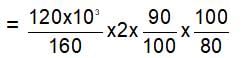Mock Test- 4 - Class 12 MCQ
30 Questions MCQ Test - Mock Test- 4
The hydrolyis of ester is slow in the beginning and become faster after some time, this is due to :
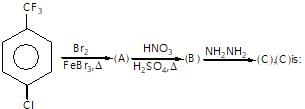
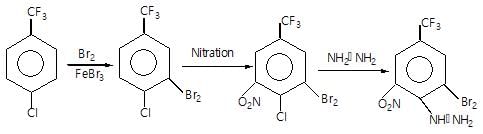
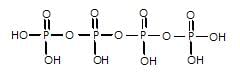
Which of the following compound gives only oxy acid as a final roduct by the hydrolysis at room temperature?
For an ideal solution containing two volatile miscible liquids A and B following graph is given:
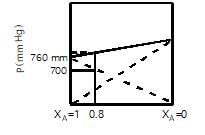
The vapour pressure of pure B (in mm of Hg) is :
Which of the following gas is responsible for ozone hole/ozone layer depletion?
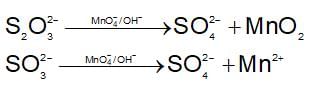
The change in Gibbs free energy of the cell in which following reaction takes place
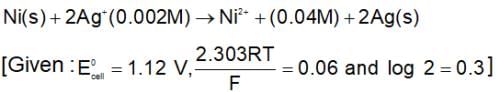
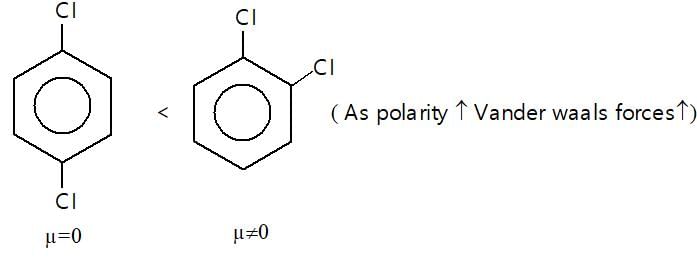
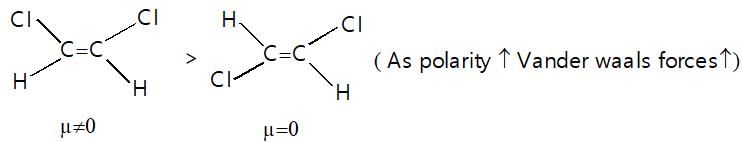
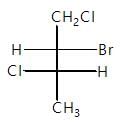
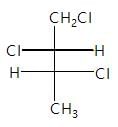
Which of the following represents incorrect order of Vander waal force?
For the reaction A+B= Product, Rate law is given as rate = K[A]1/2 [B]2. The molecularity of the reaction is:
If white bauxite is treated with concentrated solution of sodium hydroxide then SiO2 present in bauxite will :
The concentration of Ni2+ ions in a NiS solution is 2x10-6 M. If Ksp of NiS is 1.4 x 10-14 then which of the following will not cause the precipitation of NiS.
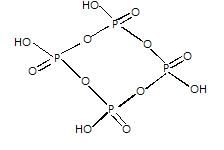
A compound forms HCP structure. The total number of tetrahedral voids present in 2 mol of it will be-
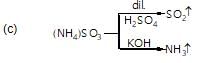
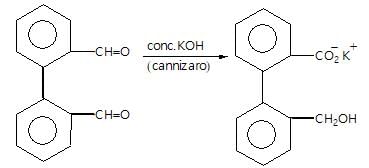
Which of the following compounds will not produce a compound with offensive smell when treated with CHCI3/KOH?
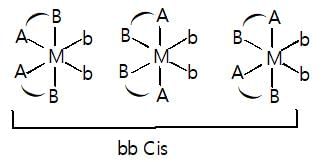
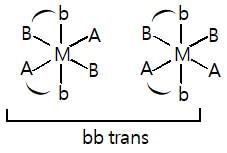
Total number of geometrical isomers of diamminebis(glycinato)platinum(IV) chloride is:
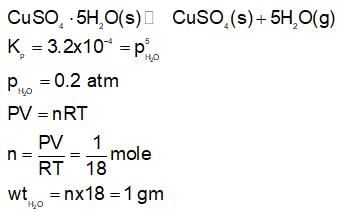
At 450 K. Calculate the mass of H2O present (in gm) in a 10 litre closed vessel which contains only CuSO4.5H2O crystal initally.
Given : CuSO4 + H2O → CuSO4(s) + 5H2O(g)
Kp = 3.2 * 10-4atm5
(Take R = 0.08 atm litre/mol K)
Mr A has two correct informations about a particular orbital of hydrogen atom
 The orbital has two radial nodes. Then orbital is identified as :
The orbital has two radial nodes. Then orbital is identified as :
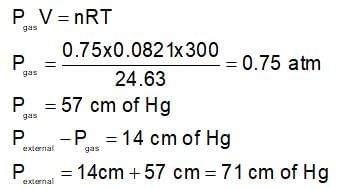
An open end manometer consists of 0.75 moles of X(g) taken in a container of volume 24.63 lit. at 300 K. The level of mercury in the open tube is found to be 14 cm lower. The height difference manometer is heated to 450 K will be:
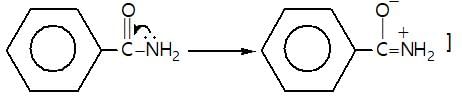
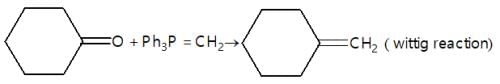
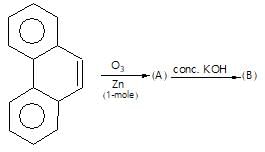
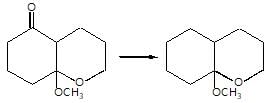
Which of the following is a good method for reduction of CO group as methioned below.
In the preparation of iron from Haematite (Fe2O3) by the reduction with carbon.
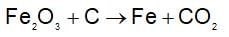
How much 80% pure iron may be produced from 120 kg of 90% pure Fe2O3?
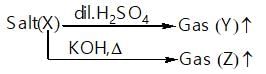
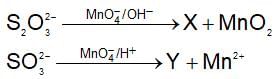
In the cyanide process for extraction of silver and gold, the cyanide act as: