Major Physical Chemistry - Chemistry MCQ
30 Questions MCQ Test - Major Physical Chemistry
Given that (at T = 298 K)
Cu(s) | Cu2+ (1.0 M) || Ag+ (1.0 M) | Ag(s) E°cell = 0.46 V
Zn(s) | Zn2+ (1.0 M) || Cu2+ (1.0 M) | Cu(s) E°cell = 1.10 V
Then, Ecell for
Zn | Zn2+ (1.0 M) || Ag+ (1.0 M) | Ag at 298 K will be
Cu(s) | Cu2+ (1.0 M) || Ag+ (1.0 M) | Ag(s) E°cell = 0.46 V
Zn(s) | Zn2+ (1.0 M) || Cu2+ (1.0 M) | Cu(s) E°cell = 1.10 V
Then, Ecell for
Zn | Zn2+ (1.0 M) || Ag+ (1.0 M) | Ag at 298 K will be
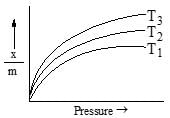
The following adsorption isotherm for a gas at adsorption surface. The correct order of temperature

Which of the following decay with the change in spin multiplicity is known as ISC (Inter System Crossing)?
92U238 emits 8α and 6β- particles. The n/p ration in daughter nucleus will be?
For a cell reaction, consider the following equations:
A + B ⇌ C + D 
2A + 2B ⇌ 2C + 2D 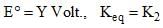
A complex containing K+, Pt(IV) and Cl- is 100% ionized giving i = 3. Thus, complex is?
Adding heat to a solid and liquid in equilibrium will cause the:
Calculate the lattice energy of KCl at 298 K. Given that:
Sublimation energy of K(s) = 89 kJ mol–1
Ionization energy of K(g) = 418 kJ mol–1
Dissociation energy of Cl2(g) = 244 kJ mol–1
Electro attachment energy of Cl(g) = –349 kJ mol–1
Formation of KCl(s) = – 437
In H-atom, electrons make transition from 5th excited state to ground state producing all possible type of photons. Calculate the number the lines in infrared region.
The standard emf of the cell, Cd(s) | CdCl2(aq) (0.1 M) || AgCl(s) | Ag(s) in which the cell reaction, is, 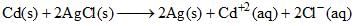 is 0.6915 V at 0°C and 0.6753 V at 25°C. The DH° of the reaction at 25°C is:
is 0.6915 V at 0°C and 0.6753 V at 25°C. The DH° of the reaction at 25°C is:
For a equilibrium H2O(s) → H2O(l) which of the following statements is true:
At low pressure if RT = 2(aP)1/2, then, the volume occupied by a real gas is?
The C-H stretching frequency of an alkane is 2900 cm-1. Calculate the value of C-D frequency (cm-1):
Given
(i) Zn + 4NH3 → Zn(NH3)42+ + 2e–, E° = 1.03 V
(ii) Zn → Zn2+ + 2e–, E° = 0.763 V
The formation constant of the complex Zn(NH3)42+ is approximately
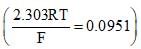
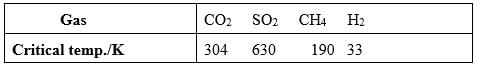
On the basis of data given below predict which of the following gases shows least adsorption on a definite amount of charcoal?
The calomel electrode and quinhydrone electrode are reversible with respect to which ion respectively:
At 30°C the solubility of Ag2CO3 (Ksp = 8 × 10–12) would be greatest in one litre of:
For an enzyme catalyzed reaction, the reaction rate is half of its maximum value when
The rate constant, the activation energy and Arrhenius parameter of a chemical reaction at 25oC are 3×10-4 s-1, 104.4 kJ/mol and 6.0×1014 s-1 resp.. Calculate the value of rate constant as time →Infinity is?
The degree of dissociation of SO3 is α at equilibrium pressure P0.
Kp for 2SO3(g) → 2SO2(g) + O2(g) is
Aluminum metal has a density of 2.72g/cm3 and crystallizes in lattice with an edge length of 404 pm. Which of the following alternative are correct:
The conductivity of a saturated solution of BaSO4 is 3.06 × 10–6 ohm–1 cm–1 and its equivalent conductance is 1.53 ohm–1 cm2 equiv–1. The Ksp for BaSO4 will be:
In the following reaction started only with Ag,
2A8(g) → 2A3(g) + 3A2(g) +A4(g)
mole fraction of A2 is found to 0.36 at a total pressure of 100 atm at equilibrium. The mole fraction of A8(g) at equilibrium is:
t1/2 (half-life period) of 1st order reaction is 10 min. Starting with 10 mol L–1, rate after 20 min is:
The phase diagram of CO2 and Bi differ in the sense that:
When pure water is saturated with CaCO3 and CaC2O4, the concentration of calcium ion in the solution under equilibrium is 8 × 10–5 M. If the ratio of the solubility product of CaCO3 to that of CaC2O4 is 3, what is the solubility product of CaCO3 in pure water?
1g charcoal show chemical adsorption of some acetic acid on coming in contact with 100 mL of 0.50 M acetic acid and the molarity of acetic acid is reduced to 0.49 M. If surface area of charcoal is 3.015 × 102 m2/g, the surface area of each chemical adsorbed molecule of acetic acid is:
Emf of Cd-cell is 1.018 V at 25°C. The temperature coefficient of cell is –5.2 × 10–5 VK–1. How cell temperature will change during operation?