Major Inorganic Chemistry - Chemistry MCQ
30 Questions MCQ Test - Major Inorganic Chemistry
Which of the following process is used in extractive metallurgy of magnesium?
Silica gel contains [CoCl4]2– as an indicator when activated, silica gel becomes dark blue while upon absorption of moisture, its color changes to pale pink. This is because:
The reagent ‘oxine’ commonly used in analytical chemistry is:
Thermal stability of hydrides of first group elements follows the order
On dissolving moderate amount of sodium metal in liquid NH3 at low temperature, which one of the following does not occur?
At pH 7, the zinc(II) ion in carbonic anhydrase reacts with CO2 to give:
NH4Cl (s) is heated in test tube. Vapours are brought in contact with red litmus paper, which changes to blue and then to red. It is because of :
Each of the compounds Pt(NH3)6Cl4, Cr(NH3)6Cl3, Co(NH3)4Cl3 and K2PtCl6 were dissolved in water to make its 0.001 M solution. The correct order of their increasing conductivity in solution is:
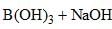 ⇌
⇌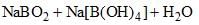
How can this reaction be made to proceed in forward direction?
In cyclophosphazenes, (NPX2)3 (X = F, Cl, Br and Me), the strength of p—N p-bond various with X in the order:
Toxicity of cadmium and mercury in the body in being reversed by proteins, mainly using the amino acid residue:
For the following reaction
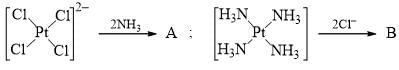
The major product A and B are respectively:
The spin-only (μS) and spin plus orbital (μS+L) magnetic moment of [CrCl6]3– are:
Choose the correct option for carbonyl fluoride with respect to bond angle and bond length:
Polarization is the distortion of the shape of an anion by an adjacently placed cation. Which of the following statement is correct:
Correct order of P—P bond length in the following compound is:





















