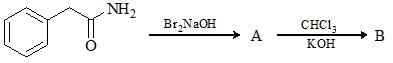IIT JAM Chemistry Mock Test 2 - Chemistry MCQ
30 Questions MCQ Test - IIT JAM Chemistry Mock Test 2
Which of the following set of ions/ molecules is isoelectronic and structural?
The hybridized state of carbon in diamond, graphite, fullerene and acetylene are:
If the molality of dilute solution is doubled, the value of molal depression constant (Kf) will be:
In which pair of ions both the species contains S—S bond?
The equilibrium constants of the following are:
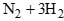
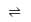

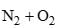
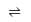
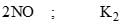

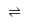

The equilibrium constant (K) of the reaction:
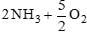
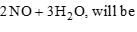
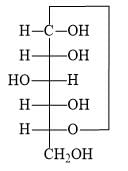
The correct Haworth structure of the following Fischer projections is:
Which of the following shows the correct decreasing order of solvolysis with aqueous ethanol?
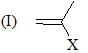
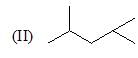
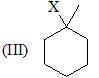

The correct choice is:
Which one of the following order of the carbonates is correct for their decomposition temeprature:
For an inverse spinal, AB2O4, the A and B are respectively, can be
If x2 + x – 1 = 0, what is the value of x4 + x – 4?
The spacing between the two adjacent lines of microwave spectrum of H35Cl is 6.35×1011 Hz. Given that the bond length of D35Cl is 5% greater than that of H35Cl, the corresponding spacing for D35Cl is?
According the consequence of the second law of thermodynamics (symbols have their usual meaning)
The correct statement regarding RNA and DNA respectively is:
A catalyst used for the polymerization of olefin is:
The reaction of PCl3 with PhLi in 1:3 molar ratio yields X as one of the products, which on further treatment with CH3I gives Y. The reaction of Y with n-BuLi gives product Z. The products X, Y and Z are resp:
For radioactive isotope I131, the time required for 50% disintegration is 8 days. The time required for 99.9 % disintegration of 5.5g of I131 is ___?
Consider the following cell reaction:
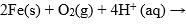

At [Fe2+] = 10–3 M, P(O2) = 0.1 atm and pH = 3, the cell potential at 25°C is:
It the radii of A+ and B– are 95 pm and 181 pm respectively, the coordination number of A+ will be?
For low partial pressure of ozone (O3), the adsorption of ozone on graphite surface is fully dissociative in nature and follows Langmuir isotherm. Under these conditions, if the dependency of surface coverage of graphite (ᴓ) on partial pressure of ozone (PO3) is given by (ᴓ) proportional to (PO3)x, the value of ‘x’ is?
The reaction of BCl3 with NH4Cl gives product A which upon reduction by NaBH4 gives product B. Product B upon reacting with HCl affords compound C which is:
If the pressure P(system) is greater than the P(surroundings), then:
If ‘I’ is the intensity of absorbed light and ‘C’ is the concentration of AB for the photochemical process 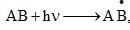 the rate of formation of
the rate of formation of 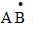 is directly proportional to:
is directly proportional to:
According to Eyring transition state theory for a bimolecular reaction, the activated complex has
The major product formed in the following reaction is
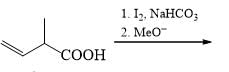
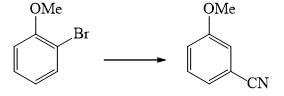
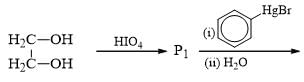
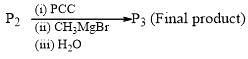
Which of the following is incorrect for the final product of the given sequence of reaction:
Major product was analyzed in IR and 1H NMR spectroscopy and following data was obtained:
IR: 1747 cm–1, 1710 cm–1
1H NMR: 1.28 (3H, t, J = 7Hz), 2.21 (3H, s), 3.24 (2H, s), 4.2 (2H, q, J = 7Hz)
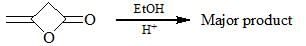
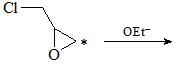
The correct set of reagents for the following conversion is: