Test: Matter - 1 - Grade 5 MCQ
15 Questions MCQ Test - Test: Matter - 1
The forces of attraction between molecules are greatest in the _________ state.
The state of matter which has no fixed shape but has definite volume is the
The intermolecular space between molecules is maximum in a _______ state.
Substance 'P' does not have a fixed volume. It occupies the total space of the container and it can be compressed. What is 'P' likely to be?
What happens to the intermolecular space when a solid is heated and changes into a liquid?
When we heat a substance in solid form, it changes into a liquid and then into gas. This shows that by heating, the space occupied by the substance generally:
In the table given below, states of matter, their properties and examples are represented by letters W, X, Y, Z.
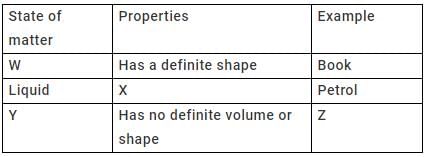
Q. Carbon dioxide can be placed in the place of letter________.



















