O.P Tandon Test: Haloalkanes & Haloarenes - JEE MCQ
30 Questions MCQ Test - O.P Tandon Test: Haloalkanes & Haloarenes
(i) CH3 – CH2 – Br + NaOH 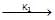 CH3 – CH2 – OH + NaBr → reaction ... (i)
CH3 – CH2 – OH + NaBr → reaction ... (i)
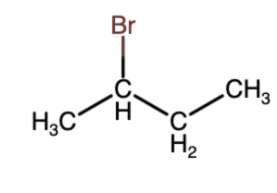
(ii) 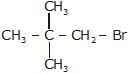 + NaOH
+ NaOH 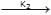 (CH3)3 C – CH2 – OH + NaBr → reaction ... (ii)
(CH3)3 C – CH2 – OH + NaBr → reaction ... (ii)
K1 & K2 are rate constant for above reaction correct relation is
Free energy profile for given reaction is
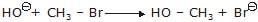
Which of the following alcohols will yield the corresponding alkyl chloride on reaction with concentrated HCl at room temperature?
Which of the following ethers is unlikely to be cleaved by hot conc. HBr ?
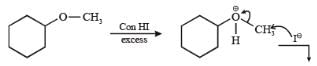
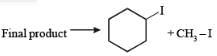
The reaction of CH3OC2H5 with one mole HI gives
Under identical conditions, solvolysis of which of the following substrates would lead to maximum racemization ?
The reaction of SOCl2 on alkanols to form alkyl chlorides gives good yields because
Which of the following alcohol shows fastest reaction with HI ?
Arrange the following compounds in order of decreasing rate of hydrolysis for SN1 reaction
(I)
(II)
(III)
(IV)
For the given reaction ;
Which substrate will give maximum racemisation
Which of these dehydrates most readily when reacts with conc. H3PO4.
Which of following halides gives fastest elimination reaction when it is treated with alcoholic KOH.
Which alkyl bromide will yield only one alkene upon E2 elimination ?
which is most easily dehydrohalogenated ?
Correct order of yield of Hofmann alkene in following reaction will be
X may be F, Cl Br or l
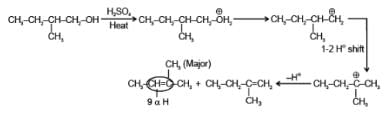
Predict the major product of the following reaction :
Which of the following compound will not undergo Nucleophillic substitution reaction.
Statement 1 : On moving 1° to 3° alkyl halide rate of E2 increases while rate of SN2 decreases
Statement 2 : E2 reaction give element effect respect to halogen.
SN1 is a two-step reaction. For each step, there has to be a transition state. Which of the following structures represent correctly the transition state of first step
Which one of the following compounds will give enantiomeric pair on treatment with HOH?
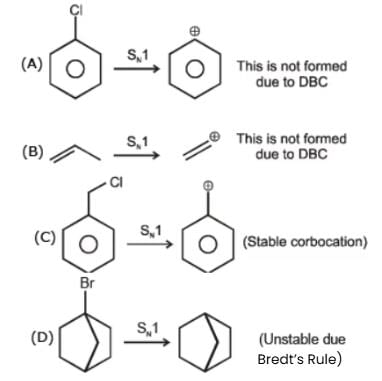
Which of the following compounds is most rapidly hydrolysed by SN1 mechanism
Which will give white ppt. with AgNO3 ?
Morrison & Boyd Test: Haloalkanes & Haloarenes
The main difference between C – X bond of a haloalkane and a haloarene is