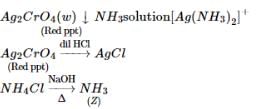Test: d & f-Block Elements - 2 - NEET MCQ
30 Questions MCQ Test - Test: d & f-Block Elements - 2
(T) imparts violet colour 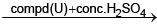 (V) Red gas
(V) Red gas 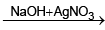 (W) Red ppt.
(W) Red ppt.
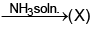
(W) Red ppt. 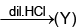 white ppt.
white ppt.
(U) 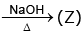 gas (gives white fumes with HCl)
gas (gives white fumes with HCl)
sublimes on heating Identify (T) to (Z).
The number of moles of acidified KMnO4 required to convert one mole of sulphite ion into sulphate ion is-
N2(g) + 3H2 (g) 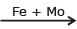 2NH3(g) ; Haber's process, Mo is used as -
2NH3(g) ; Haber's process, Mo is used as -
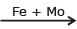 2NH3(g) ; Haber's process, Mo is used as -
2NH3(g) ; Haber's process, Mo is used as -Potash alum is a double salt, its aqueous solution shows the characteristics of-
Addition of non-metals like B and C to the interstitial sites of a transition metal results the metal-
The correct statement(s) about transition elements is/are-
An ornament of gold having 75% of gold, it is of .............. carat.
The ionisation energies of transition elements are-
Transition elements having more tendency to form complex than representative elements (s and p-block elements) due to-
During estimation of oxalic acid Vs KMnO4, self indicator is-
The metal(s) which does/do not form amalgam is/are-
Which of the following statements concern with transition metals ?
The highest oxidation state among transition elements is-
A compound of mercury used in cosmetics, in Ayurvedic and Yunani medicines and known as Vermilon is-
Acidified chromic acid
X and Y are - (blue colour)
Transition elements are usually characterised by variable oxidation states but Zn does not show this property because of-
(NH4)2Cr2O7 (Ammonium dichromate) is used in fire works. The green coloured powder blown in air is-
The d-block element which is a liquid at room temperature, having high specific heat, less reactivity than hydrogen and its chloride (MX2) is volatile on heating is-
Iron becomes passive by..................due to formation of ................
Bayer's reagent used to detect olifinic double bond is-