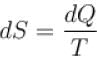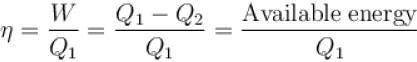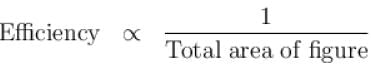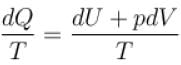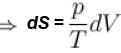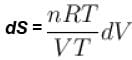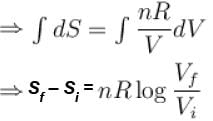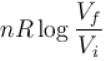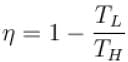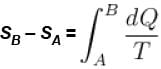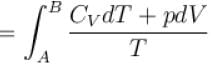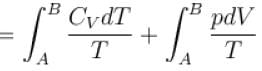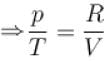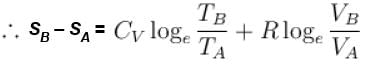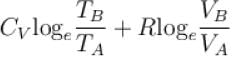Second Law Of Thermodynamics MCQ Level – 2 (Part - 1) - Physics MCQ
10 Questions MCQ Test - Second Law Of Thermodynamics MCQ Level – 2 (Part - 1)
The property of a working substance which increases or decreases as the heat is supplied or removed in a reversible manner is known as.
Select one:
Select one:
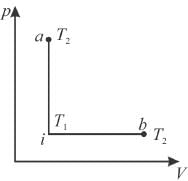
An ideal gas has temperature T1 at the initial state i shown in the p-V diagram here. The gas has a higher temperature T2 at final states a and b, which it can reach along the path shown. The entropy change along the path to state a is
Select one:

Select one:
The T-S diagram of two cycles for the operation of an engine are shown in figure below.
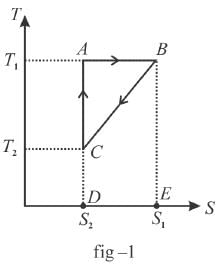
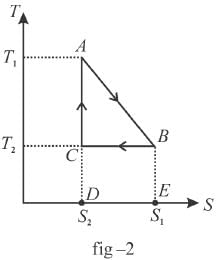
The numerical values of the parameters T1 , T2 , S1 and S2 in the two figures are the same. Which cycle has greater efficiency?
Select one:


The numerical values of the parameters T1 , T2 , S1 and S2 in the two figures are the same. Which cycle has greater efficiency?
Select one:
The difference in entropy between a state of volume Vi and a state of volume of Vf (temperature and number of molecules remaining constant) is equal to.
Select one:
The efficiency of the Carnot cycle may be increased by.
Select one:
Kelvin-Plank’s law deals with
Select one:
In a reversible process, entropy of the system.
Select one:
1gm of perfect gas at volume VA , pressure pA and temperature TA changes from state A to state B when volume is VB, pressure is pB and temperature TB. the change in entropy is.
Select one:
Change in entropy depends
Select one:
In an irreversible process, the entropy of the universe.
Select one: