Test: f-Block Elements (Actinides) - II - NEET MCQ
25 Questions MCQ Test - Test: f-Block Elements (Actinides) - II
Only One Option Correct Type
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Which one of the following is an electronic configuration of thorium?
Which of the following is general electronic configuration of actinides?
The actinoids Exhibit more member of oxidation states in general than the lanthanoids. This is because
Identify the incorrect statement among the following.
Which of the following is not an actinide?
The compound used in enrichment of uranium for nuclear power plant is
Which one of the following shows oxidation state upto + 7?
The general electronic configuration of actinides is (n - 2) f1-14 (n - 1) d0-1 ns2.
Which one of the following actinides has one electron in 6d-orbital?
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Commonly used nuclear fuels are
Which species have half-filled 5f subshell?
Comprehension Type
Direction (Q. Nos. 16 and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Rutherford and Soddy found that every uranium ore that they examined contained minute quantities of radium and an appreciable amount of non-radioactive lead. From this, they concluded that uranium is the starting substance in a series of radioactive transformations which pass through radium and ultimately ends with stable lead. These transformations are accompanied by spontaneous emissions of alpha and beta particles resulting in the formation of new atoms. All the nuclei from the initial element to the final stable element constitute a series called disintegration series. Mass number changes by 4 units per each alpha particle emission.
There are four disintegration series. They are
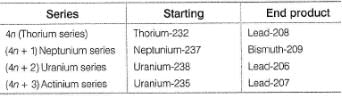
Q.
The number of alpha and beta particles emitted in the nuclear reaction are
Rutherford and Soddy found that every uranium ore that they examined contained minute quantities of radium and an appreciable amount of non-radioactive lead. From this, they concluded that uranium is the starting substance in a series of radioactive transformations which pass through radium and ultimately ends with stable lead. These transformations are accompanied by spontaneous emissions of alpha and beta particles resulting in the formation of new atoms. All the nuclei from the initial element to the final stable element constitute a series called disintegration series. Mass number changes by 4 units per each alpha particle emission.
There are four disintegration series. They are

Q.
Lead isotope is the end product in all disintegration series except
Matching List Type
Direction (Q. No. 18) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.

One Integer Value Correct Type
Direction (Q. Nos. 19-23) This section contains 5 questions. When worked out wiil result in an integer from 0 to 9 (both inclusive).
Q.
The number of electrons present in 5f subshell of berkelium are
The number of electrons present in 5f subshell of Th4+ ion are
Number of elements that show + 6 oxidation state among Th, U, Np, Pu, Am, Cm, Bk, Cf, Es, Fm are
The number of unpaired electrons in the element with atomic number 100 are
Pb, in this transformation, number of alpha particles (α) liberated
Statement Type
Direction (Q. Nos. 24 and 25) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : Actinides show a large number of oxidation state whereas lanthanides show a limited number of oxidation state.
Statement Il : Energy gap between 4f, 5d and 6s subshells is small whereas that between 5f, 6d and 7s subshell is large.
Statement I : Actinides form relatively less stable complexes as compared to lanthanides.
Statement II : Actinides can utilise their 5f -orbitals along with 6d-orbitals in bonding but lanthanides do not use their 4f-orbitals for bonding.



















