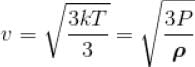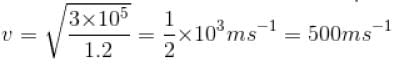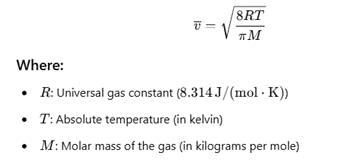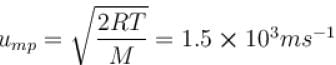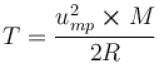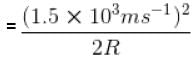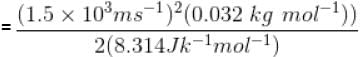Kinetic Theory Of Gases MCQ Level – 1(Part - 1) - IIT JAM MCQ
10 Questions MCQ Test - Kinetic Theory Of Gases MCQ Level – 1(Part - 1)
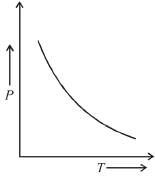
At a constant pressure, which graph represents the variation of the density of an ideal gas with the absolute temperature T?
Four molecules of a gas are having speed of 1, 4, 8 and 16 ms-1. The root mean square velocity of the gas molecules is
Select one:
Select one:
The root mean square velocity of a gas molecule is 300 m/s. What is the r.m.s velocity of a molecule of gas with twice the molecular weight and half the absolute temperature?
The energy density u/V of an ideal gas is related to its pressure p as.
Select one:
A gas behaves as an ideal gas at ___________
V versus T curves at constant pressure P1 and P2 for an ideal gas as shown in figure. Here
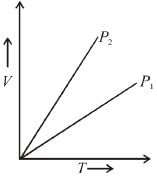
Select one:
The density of air at a Pressure of 105 Nm-2 is 1.2 kgm-3. Under these condition, the root mean square velocity of the air molecule in ms-1 is
Select one:
Formula for average speed of a gas having M molecular mass.
Select one:
For O2 gas molecule, the most probable speed at temperature T is equal to 1.5 × 103 ms-1 , then temperature T is
Select one:
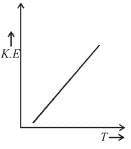
Which of the following graphs best represents the relationship between absolute temperature of a gas and the average kinetic energy of the gas molecules?
Select one:


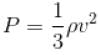
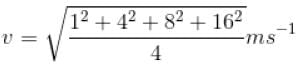
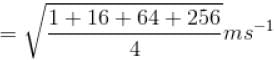
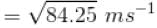

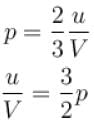
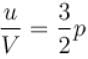
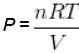
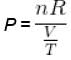
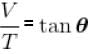
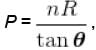 where tanθ is the slope of the graph
where tanθ is the slope of the graph