Retro (Past 13 Year) JEE Main (D Block Elements) - JEE MCQ
24 Questions MCQ Test - Retro (Past 13 Year) JEE Main (D Block Elements)
Which of the following arrangements does not represent the correct order of the property stated against it ?
(JEE Main 2013)
Four successive members of the first row transition elements listed below with atomic numbers. Which one of them is expected to have the highest 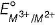 value ?
value ?
(JEE Main 2013)
Iron exhibits +2 and +3 oxidation states. Which of the following statements about iron is incorrect?
(AIEEE 2012)
In context of the lanthanides, which of the following statements is not correct?
(AIEEE 2011)
The outer electronic configuration of Gd (Atomic number 64) is
(AIEEE 2011)
The correct order of values with negative sign for the four successive elements Cr, Mn, Fe and Co is
(AIEEE 2010)
Knowing that the chemistry of lanthanides (Ln) is dominated by its +3 oxidation state, which of the following statements is incorrect?
In context with the transition elements, which of the following statements is incorrect?
(AIEEE 2009)
Larger number of oxidation states are exhibited by the actinides than those by the lanthanides, the main reason being
(AIEEE 2008)
Identify the incorrect statement among the following.
(AIEEE 2007)
The actinides exhibit more number of oxidation states in general than the lanthanides. This is because
(AIEEE 2007)
Lanthanides contraction is caused due to
(AIEEE 2006)
On heating, mixture of Cu2O and CU2S will give
(AIEEE 2005)
Calomel (Hg2CI2)on reaction with ammonium hydroxide gives
(AIEEE 2005)
Cerium (Z = 58) is an important member of the lanthanides. Which of the following statements about cerium is incorrect?
(AIEEE 2004)
Of the following outer electronic configurations of atoms, the highest oxidation state is achieved by which one of them ?
(AIEEE 2004)
Which of the following groups of transition metals is called coinage metals?
(AIEEE 2003)
Which one of the following nitrates will leave behind a metal on strong heating?
(AIEEE 2003)
For making good quality mirrors, plates of float glass are used. These are obtained by floating molten glass over a liquid metal which does not solidify before glass. The metal used can be
(AIEEE 2003)
Which one of the following statements is correct?
(AIEEE 2003)
A red solid is insoluble in water. However, it becomes soluble if some Kl is added to water. Heating the red solid in a test tube results in liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is
(AIEEE 2003)
What would happen when a solution of potassium chromate is treated with an excess of dilute nitric acid?
(AIEEE 2003)
Most common oxidation states of Ce (cerium) are
(AIEEE 2002)
Which of the following ions has the maximum magnetic moment?
(AIEEE 2002)



















