Test: Different Methods of Expressing Concentration of Solutions - JEE MCQ
30 Questions MCQ Test - Test: Different Methods of Expressing Concentration of Solutions
Only One Option Correct Type
This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Which of the following concentration factors is affected by change in temperature?
This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
6.02 x1020 molecules of urea are present in 100 mL of its solution. The concentration of urea solution is (N0 = 6.02 x 1023 mol-1)
[AIEEE 2004]
Glucose solution is one molal. Glucose present in 1 kg glucose solution is
The molality of a urea solution in which 0.0100 g of urea (NH2CONH2) is added to 0.300 dm3 of water at STP is
[AIEEE 2011]
A solution that is 20% ethanol by volume is found to have a density of 0.977 g/mL. Density of ethanol is 0.789 g/mL. Thus, mass per cent of ethanol solution is
A solution of NH4CI is prepared by dissolving 95 g of NH4CI in 200 g of H2O at 60° C. What is mass per cent when the solution is cooled to 20° C based on this
Commercial concentrated nitric acid is 15.6 M. To prepare 10 L of 6.0 M nitric acid from it,
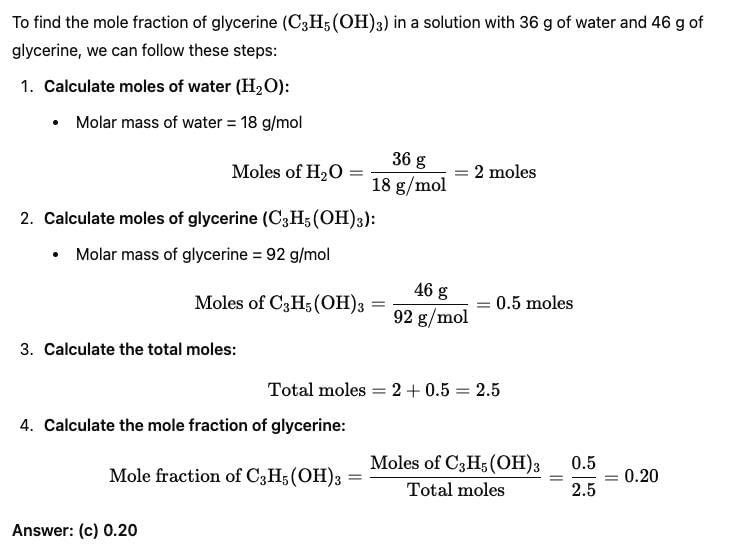
Mole fraction of C3H5(OH)3 in a solution of 36 gm of water and 46 gm of glycerine is
AgNO3 sample is 85% by mass. To prepare 125 mL of 0.05 M AgNO3 solution, AgNO3 sample required is
Hardness of a water sample is 200 ppm CaCO3, Thus, molarity of CaCO3 is
100 mL of aqueous solution of 0.01 M CaCI2 is evaporated to dryness when 0.15 g of residue is obtained. Thus, impurity present is
At 25 ° C, the density of 18 MH2SO4 is 1.8 g cm3. Thus, mass percentage of H2SO4 in aqueous solution is
Laboratory ammonia is 14.8 M NH3(ag)with a density of 0.8980 g/mL. Mole fraction of NH3 in this solution is
An aqueous solution is 34% H3P04 by mass and has density 1.209 g mL -1 Molarity (I), molality (II) and normality (III) respectively, are
If one assumes the volumes are additive, what is in a solution obtained by mixing 275 mL of 0.200 M KN03, 325 mL of 0.40 M Mg(N03)2 and 400 mL H20?
How many grams of water would you add to 1.38 moles of CH3OH in 1 kg water to reduce the molality to 1.00 molal CH3OH(aq) ?
Three solutions have been provided.
I. 3 g HCI in 1 kg H20.
II. 2 L HCI(g) and 1 L H20 at room temperature.
III. Aqueous solution of HCI with
Solutions containing same number of moles of HCI is/are
Which of the following aqueous solutions has the highest concentration of Na+?
pH of Ba(OH)2 solution is 12. Number of millimoles present in 100 mL of Ba(OH)2 solution is
We have
I. 25 mL of 1 M NaOH
II. 10 mL of 0.50 M NaCI
On mixing the two solutions, molar concentrations of Na+, OH- and Cl- respectively, are
A ‘100 proof solution of ethanol in water consists of 50.0 mL of C2H5OH(/)and 50.0 mL of H20 (/), mixed at 16 °C . Given,
Density of H20 = 1 g mL-1
Density of C2H,OH = 0.7939 gmL-1
Density mixture = 0.9344 g mL-1
Volume of the solution is
How many grams of Mgl2 must be added to 250 mL of 0.0876 M Kl to produce a solution with [l-] = 0.10 M?
An aqueous solution is 6% methanol, CH3OH, by mass with d = 0.988 g mL-1. Thus, molarity of CH3OH in this solution is
Matching List Type
Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct
An ethanol-water solution is prepared by dissolving 10.00 mL o f ethanol (C2H5OH, d = 0.789 g mL-1) in a sufficient volume o f w ater to produce 100 mL of solution with a density, d = 0.982 g mL-1. Match the concentration term in Column I with its value in Column II and select the answer from the code
One or More than One Options Correct Type
This section contains 2 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
1 kg of aqueous solution has 0.40 kg NaOH. Thus, this solution is
Mole fraction of ethanol in ethanol-water solution is 0.25. Thus, this solution is
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
Passage
A handbook gives the concentration of water in a 2.772 M aqueous solution of NaOH as 998.0 g L-1.
Q. Molality of solution is
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
Passage
A handbook gives the concentration of water in a 2.772 M aqueous solution of NaOH as 998.0 g L -1.
Q.
Molality of solution is
One Integer Value Correct Type
This section contains 1 questions, when worked out will result in an integer value from 0 to 9 (both inclusive)
Q. Certain brine solution has 3.87% NaCI by mass with density 1.036 g mL-1. How many millilitre of this solution should be evaporated to obtain 0.32 g of NaCI?
A 5.2 molal aqueous solution of methyl alcohol (CH3OH) is supplied. What is the mole fraction of methyl alcohol in the solution?