Test: Isomerism In Coordination Compounds- II - JEE MCQ
25 Questions MCQ Test - Test: Isomerism In Coordination Compounds- II
Only One Option Correct Type
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q. Which type of isomerism is shown by [Co(NH3)4 Br2CI]?
The complex that exists as a pair of enantiomers is
Which of the following will have three stereoisomeric form ?
i. [Cr(NO3)3(NH3)3]
ii. K3[Co(C2O4)3]
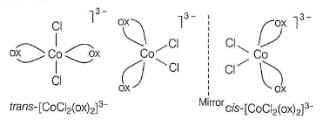
iii. K3[Co(C2O4)2CI2]
iv. [Co(en)2CIBr]
ii. K3[Co(C2O4)3]
iii. K3[Co(C2O4)2CI2]
iv. [Co(en)2CIBr]
A coordination compound of cobalt has the molecular formula containing five ammonia molecules, one nitro group and two chlorine atoms for one cobalt atom. One mole of this compound produces three ions in an aqueous solution. The aqueous solution on treatment with an excess of AgNO3 gives two moles of AgCI as a precipitate. The formula of the complex and the isomerism shown by this is
Which of the following will give maximum number of isomers?
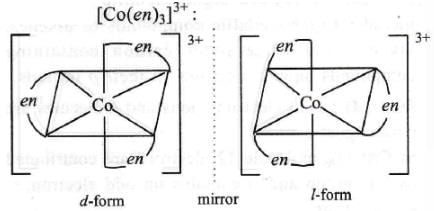
Which of the following coordination compounds would exhibit optical isomerism?
Which one of the following is expected to exhibit optical isomerism?
(en = ethylenediamine)
How the isomeric complexes [Co(NH3)6] [Cr(NO2)6] and [Cr(NH3)6] [Co(NO2)6] can be distinguished from one another by
The complexes [Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6] are the examples of which type of isomerism?
[AIEEE 2011]
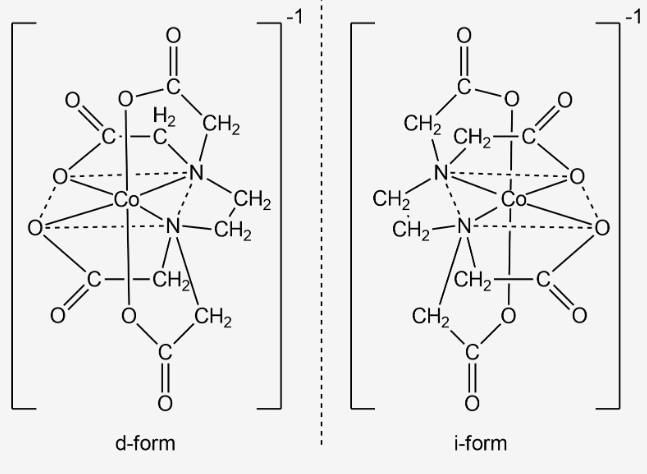
The isomerism shown by [Co(EDTA)]- ion is
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
[Co(NH3)4 (NO2)2]Cl exhibits
The complex [Co(NH3)5Br] SO4 and [Co(NH3)5SO4] Br can be identified by
The pair(s) of coordination complexes/ions exhibiting the same kind of isomerism is/are
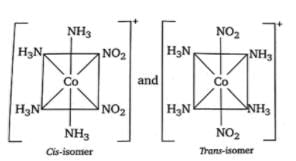
Which of the following complexes show geometrical isomerism?
The compound(s) that exhibit(s) geometrical isomerism is/are
Comprehension Type
Direction (Q. Nos. 16 and 17) This section contains a paragraph, each describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
A metal complex having the composition Cr(NH3)4CI2Br has been isolated in two forms A and B. The form A reacts with AgNO3 to give a white precipitate readily soluble in dilute aqueous ammonia whereas B gives a pale yellow precipitate soluble in cone, ammonia.
Q.
The formula of complex A
A metal complex having the composition Cr(NH3)4CI2Br has been isolated in two forms A and B. The form A reacts with AgNO3 to give a white precipitate readily soluble in dilute aqueous ammonia whereas B gives a pale yellow precipitate soluble in cone, ammonia.
Q.
The isomerism shown by complex A is
Matching List Type
Direction (Q. Nos. 18 and 19) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d) out of which one is correct.
Q.
Match the Column I with Column II and mark the correct option from the codes given below.


Match the Column I with Column II and mark the correct option from the codes given below.
One Integer Value Correct Type
Direction (Q. Nos. 20 -24) This section contains 5 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
The total number of isomers expected for [Pt(NCS)(en)2]2+ are
Number of isomers possible for [Co(NH3)5SCN]CI are
[Fe(EDTA)]- exhibits optical isomerism. In this complex, the number of rings formed are
The number of geometrical isomers possible for the complex [CoCI2Br2]- are
The total number of possible isomers for the complex compound
[CuII(NH3)4] [PtIICI4] are
Statement Type
Direction (Q. No. 25) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : Ambidentate ligands lead to linkage isomerism.
Statement II : The ionisation sphere is different in different linkage isomers.