Retro (Past 13 Year) IIT-JEE Advanced (Coordination Compounds) - Class 12 MCQ
27 Questions MCQ Test - Retro (Past 13 Year) IIT-JEE Advanced (Coordination Compounds)
Match each coordination compound in Column I with an appropriate pair of characteristics from Column II and select the correct answer using the codes given below the columns
(en = H2NCH2CH2NH2; atomic numbers : Ti = 22; Cr = 24; Co = 27; Pt = 78)
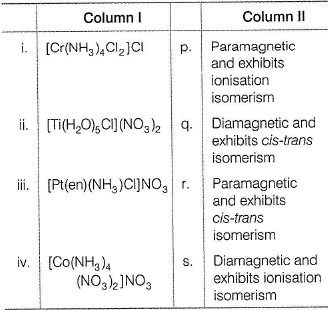
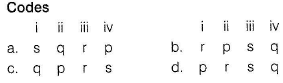
(2014 Adv. Matching Type)
EDTA4- is ethylenediaminetetraacetate ion. The total number of N—Co—O bond angles in [Co(EDTA)]- complex ion is
(2013Adv. Integer Type)
Consider the following complex ions, P, Q and R.
P = [FeF6]3- Q = [V(H2O)6]2+ and R = [Fe(H2O)6]2+
The correct order of the complex ions, according to their spinonly magnetic moment values (in BM) is
(2013 Adv. Only One Option Correct Type)
P = [FeF6]3- Q = [V(H2O)6]2+ and R = [Fe(H2O)6]2+
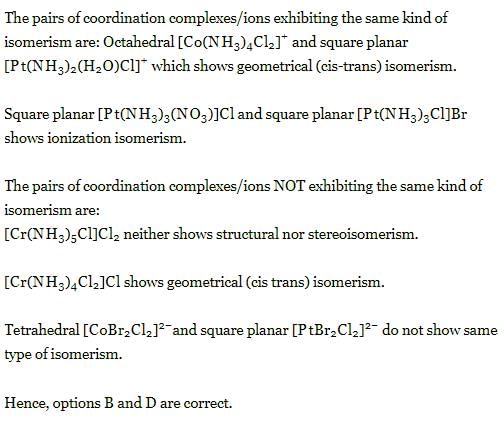
The pair(s) of coordination complexes/ions exhibiting the same kind of isomerism is(are)
(2013 Adv. One or More than One Options Correct Type)
As per IUPAC nomenclature, the name of the complex [Co(H2O)4(NH3)2]CI3 is
(2012, Only One Option Correct Type)
NiCI2{P(C2H5)2(C6H5)}2 exhibits temperature dependent magnetic behaviour (paramagnetic/diamagnetic) the coordination geometries of Ni2+ in the paramagnetic and diamagnetic states respectively are
(2012, Only One Option Correct Type)
Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl-, CN- and H2O respectively, are
(2011, Only One Option Correct Type)
Among the follow ing com plexes (K-P),
K3[Fe(CN)6] (K), [Co(NH3)6]CI3 (L) Na3[Co(ox)3] (M), [Ni(H2O)6]CI2 (N), K2 [Pt(CN)4](O) and [Zn(H2O)6] (NO3)2 (P) the diamagnetic complexes are
(2011, Only One Option Correct Type)
The volume (in mL) of 0.1 M AgNO3 required for complete precipitation of chloride ions present in 30 mL of 0.01 M solution of [Cr(H2O)5CI]CI2, as Silver Chloride is Close to
(2011, Integer Type)
Total number of geometrical isomers for the complex [RhCI(CO)(PPh3)(NH3)] is
(2010, Integer Type)
The ionisation isomer of [Cr(H2O)4CI(NO2)]CI is
(2010, Only One Option Correct Type)
The correct structure of ethylenediaminetetraacetic acid (EDTA) is
(2010, Only One Option Correct Type)
The Complex showing a spin-only magnetic moment of 2.82 B
(2010, Only One Option Correct Type)
The Compound(s) that exhibit(s) geometrical isomerism is(are)
(2009, One or More than One Options Correct Type)
The spin only magnetic moment value (in Bohr magneton units) of Cr(CO)6 is
(2009, Only One Options Correct Type)
Both [Ni(CO)4] and [Ni(CN)4]2- are diamagnetic. The hybridisation of nickel in these complexes respectively, are
(2008, Only One Option Correct Type)
The IUPAC name of [Ni(NH3)4][NiCI4] is
Among the following, the coloured compound is
Direction : This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statment I : The geometrical isomers of the complex [M(NH3)4CI2]are optically inactive.
Statment II : Both geometrical isomers of the complex [M(NH3)4CI2] possess axis of symmetry.
Statement I : [Fe(H2O)5NO]SO4 is paramagnetic.
Statement II : Fe in [Fe(H2O)5NO]SO4 has three unpaired electrons.
(2008, Statement Type)
Match the complexes in Column I with their properties listed in Column II.
(2007, Matching Type)
Among the following metal carbonyls,the C—O bond order is lowest in
(2007, Only One Option Correct Type)
If the bond length of CO bond in carbon monoxide is 1.128 Å, then what is the value of CO bond length in Fe(CO)5 ?
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
The coordination number of Ni2+ is 4.
NiCI2 + KCN (excess) → A (cyano complex)
NiCI2 + conc. HCI (excess) → B (chloro complex)
Q.
The IUPAC name of A and B are
(2006, Comprehension Type)
The coordination number of Ni2+ is 4.
NiCI2 + KCN (excess) → A (cyano complex)
NiCI2 + conc. HCI (excess) → B (chloro complex)
Q.
Predict the magnetic nature of A and B.
The coordination number of Ni2+ is 4.
NiCI2 + KCN (excess) → A (cyano complex)
NiCI2 + conc. HCI (excess) → B (chloro complex)
Q.
The hybridisation of A and B
Which kind of isomerism is shown by Co(NH3)4Br2Cl ?
(2005, Only One Option Correct Type)