Test: Extraction of Crude Metals From Concentrated Ores (Old NCERT) - JEE MCQ
25 Questions MCQ Test - Test: Extraction of Crude Metals From Concentrated Ores (Old NCERT)
Only One Option Correct Type
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Which of the following is not correctly matched ?
The purpose of adding cryolite is
In Mac Arthur Forest method, silver is extracted from the solution of Na[Ag(CN)2] by the use of
In the extraction of copper, the metal formed in the Bessem er’s convertor is due to the reaction
The nitrate of which metal leaves metallic globule on strongly heating ?
In the extraction of gold, the cyanide solution acts as a
The matte is impure substance obtained during the extraction of
During the extraction of gold the following reactions takes place
Au + CN- + H2O 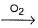 X,
X,
X + Zn → Y + Au
Identify the reaction that does not take place in a blast furnace
Roasting of sulphides gives the gas X as a byproduct. This is a colourless gas with choking smell of burnt sulphur and causes great damage to respiratory organs as a result of acid rain. Its aqueous solution is acidic, acts as reducing agent and its acid is never isolated. The gas X is
One or More than One Options Correct Type
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
In the following processes, which are not sulphatising roasting ?
In the extraction of copper from its sulphide ore, the metal is finally obtained by the reduction of cuprous oxide with
Which is correctly matched?
Which of tlfe following metal sulphides are converted into metals by self reduction?
Which of the following are correct process ?
Comprehension Type
Direction (Q. Nos. 16 and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Copper is extracted from copper pyrites. After roasting, the ore is mixed with silica and coke and then smelted in a blast furnace. The matte obtained from the blast furnace is charged into silica lined convertor. Some silica is also added and a hot air blast is blown into the mixture to obtain blister copper which is purified by electro refining.
Q.
The chemical formula of copper pyrites is
Copper is extracted from copper pyrites. After roasting, the ore is mixed with silica and coke and then smelted in a blast furnace. The matte obtained from the blast furnace is charged into silica lined convertor. Some silica is also added and a hot air blast is blown into the mixture to obtain blister copper which is purified by electro refining.
Q.
Coke is added during smelting
Matching List Type
Direction (Q. Nos. 18 and 19) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Q.
Match the Column I with Column II and mark the correct option from the codes given below.
Match the Column I with Column II and mark the correct option from the codes given below.
One Integer Value Correct Type
Direction (Q. Nos. 20-24) This section contains 5 questions. When worked out will result is an integer from 0 to 9 (both inclusive)
Q.
Among Ca, Pb, Sn, Ag, Mg, Au, Zn, Cu, Al, Na, Hg the number of metals extracted by pyrometallurgy are
Among Ca, Pb, Sn, Ag, Mg, Au, Zn, Cu, Al, Na, Hg, K, the number of metals extracted by electrometallurgy are
in this how many CN- ions are involved in the balanced equation?
In the electrolytic extraction of Al at cathode Al metal and at anode F2 gas is liberated. If the molar ratio of Al and F2 is 2 : x, here value of is x
In Goldschmidt alum inothermic process, therm it mixture contains ...... parts Fe2O3 and one part Al powder.
Statement Type
Direction (Q. No. 25) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : Cast iron is obtained by remelting pig iron with scrap iron and coke wing hot air blast.
Statement II : In the extraction of silver, silver is extracted as cationic complex.



















