Test: Ellingham's Diagram & Its Applications: Thermodynamic Principles of Metallurgy (Old NCERT) - JEE MCQ
25 Questions MCQ Test - Test: Ellingham's Diagram & Its Applications: Thermodynamic Principles of Metallurgy (Old NCERT)
Only One Option Correct Type
Direction : This section contains 9 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Ellingham diagram represents a graph of
ΔG° vsT plot in the Ellingham diagram slopes down for the reaction.
The following reactions take place in the blast furnace in the preparation of impure iron. Identify the reaction pertaining to the formation of the slag.
Which metal has a greater tendency to form metal oxide?
The main reaction occurring in blast furnace during the extraction of iron from haematite is
Consider the following reactions at 10000° C,
Then choose the correct statement from the following.
According to Ellingham diagrams the oxidation reaction of carbon and carbon monoxide may be used to reduce which one of the following oxides at the lowest temperature?
The minimum voltage required to electrolyse alumina in the Hall-Heroult process is [Given, 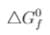 (Al2O3) = - 1520 kJ/mol and
(Al2O3) = - 1520 kJ/mol and 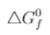 (CO2) = 394 k J/mol]
(CO2) = 394 k J/mol]
Based on the given information 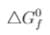 (CaO) = - 604.2 kJ/mol and
(CaO) = - 604.2 kJ/mol and 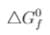 (Al2O3) = - 1582 kJ/mol, which of the following is feasible?
(Al2O3) = - 1582 kJ/mol, which of the following is feasible?
One or More than One Options Correct Type
Direction : This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
For the metallurgical process of which of the calcined ore can be reduced by carbon?
For which oxide formation ΔG° is more negative than Gr2O3?
In the extraction of iron from haematite in blast furnace reducing agent
Based on the given information, (CaO) = -604.2 kJ/mol and
(Al2O3) = - 1582 kJ/mol, which o f the following is correct?
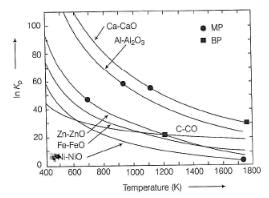
Consider the Ellingham diagram given below. Which metal(s) in the diagram can be extracted at 1100 K using coke as a reducing agent?
Comprehension Type
Direction : This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Q.
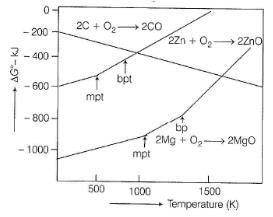
To make the reduction ZnO+ C → Zn + CO spontaneous, temperature should be
Passage
Q.
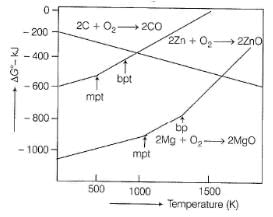
At 1100°C, which reaction is spontaneous to a maximum extent ?
Matching List Type
Direction : Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Q.
Match the Column I with Column II and mark the correct option from the codes given below.
Match the Column I with Column II and mark the correct option from the codes given below.
One Integer Value Correct Type
Direction : This section contains 5 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
In the Gibbs equation at equilibrium the value of ΔG is
The reaction 3CaO + 2AI → Al2O3 + 3Ca is non-spontaneous. The number of electrons involved are
The given reaction 2AI + Cr2O3 → Al2O3 + 2Cr is spontaneous. The number of electrons involved are
Among PbO, ZnO, CaO, Al2O3, SnO2, Cr2O3, WO3, HgO the oxides reduced by carbon are
Cu2+ + Fe → Cu + Fe2+ for this reaction to calculate ΔG° the formula ΔG° = - nFE° is used. Here, value of n is
Statement Type
Direction : This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : Carbon is good reducing agent for sulphides.
Statement II : Carbon is poor reducing agent for iron oxide.
Statement I : Carbon can reduce Fe2O3 to Fe at a temperature below 983 K.
Statement II : Iron has higher affinity toward oxygen than carbon.



















