Test: Metallurgy of Some Important Elements (Al, Zn, Au) (Old NCERT) - JEE MCQ
25 Questions MCQ Test - Test: Metallurgy of Some Important Elements (Al, Zn, Au) (Old NCERT)
Only One Option Correct Type
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
In the electrolytic refining of zinc
When compared to ΔG° for the formation of Al2O3 the ΔG° for the formation of Cr2O3 is
During the extraction of gold the following reactions take place
Au + CN- + H2O 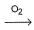 X,
X,
X + Zn → Y + Au
Here, X and Y respectively are
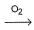 X,
X,X + Zn → Y + Au
Gold is extracted by hydrometallurgical process based on its property
During Hoope’s process for electrolytic refining of Al, the middle layer is of
Thermite is a mixture of iron oxide and
Which is an alloy of aluminium?
Which one of the following metals has the largest abundance in the earth’s crust?
The processes used in the refining of aluminium and zinc metals respectively are
In sulphatizing roasting of ZnS products are
One or More than One Options Correct Type
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Purification of alumina is called
Among the following statements the correct one is
The alloys having zinc metal are
Which is a zinc compound?
Which is not a mineral of Al?
Comprehension Type
Direction (Q. Nos. 16 and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
The chief ore of zinc is ZnS.The ore is purified by floatation process and then heated in air which converts ZnS into ZnO. ZnO can be reduced with coke in Belgian process to get zinc which is purified by electrolysis.
Alternately zinc can be extracted from calamine .This after purification by levigation, subjected to calcination to get oxide. This on reduction by Belgian method followed by electrolytic refining yields pure zinc.
Q.
Zinc oxide is converted into zinc by
Passage
The chief ore of zinc is ZnS.The ore is purified by floatation process and then heated in air which converts ZnS into ZnO. ZnO can be reduced with coke in Belgian process to get zinc which is purified by electrolysis.
Alternately zinc can be extracted from calamine .This after purification by levigation, subjected to calcination to get oxide. This on reduction by Belgian method followed by electrolytic refining yields pure zinc.
Q.
Which of the following graph in the given figure is the Ellingham diagram of ZnO?
Matching List Type
Direction (Q. Nos. 18 and 19) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Q.
Match the Column I with Column II and mark the correct option from the codes given below.
Match the Column I with Column II and mark the correct option from the codes given below.
One Integer Value Correct Type
Direction (Q, Nos. 20-24) This section contains 5 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
Gibbsite formula is Al2O3 . xH2O. Here, value of x is
Gold is soluble in aqua-regia which is cone. HNO3 and cone. HCI in 1: x ratio. Then the value of x is
Duralumin is an important alloy of aluminium .This has 95% Al and ...... % copper.
Al is amphoteric metal and liberate hydrogen by reacting with both with dilute acids and bases . 2 moles of Al metal when reacted with either acid or base, the number of moles of dihydrogen liberated is ......
During the Hall-Heroult process of preparing Al metal from purified bauxite, if 3 moles of O2 is formed at anode the number of moles of Al formed at cathode is
Statement Type
Direction (Q. No. 25) This section is based,on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : Many gem stones are impure forms of alumina (Al2O3).
Statement II : In cyanide process gold (Au) is treated with a cone, solution of sodium or potassium cyanide.














